Abstract
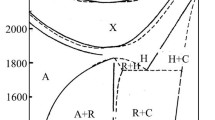
Phase equilibrium relations in the V2O3-La2O3 system were investigated by X-ray powder diffraction and metallographic techniques. Binary mixtures, prepared from high-purity V2O3 and La2O3 powders, were equilibrated at 1600° C and then arc-melted under a partial pressure of argon. The specimens were heat-treated at various predetermined temperatures for prolonged periods and the phases present were identified by reflected-light microscopy and X-ray powder diffraction. The system consists of only one binary compound LaVO3. A eutectic between V2O3 and LaVO3 was established at 1750° C and 19 mol % La2O3 and also between LaVO3 and La2O3 at 1765° C and 75 moi % La2O3. No appreciable solid solubility was detected in the system.
Similar content being viewed by others
References
H. L. Yakel, Acta Cryst. 8 (1955) 394.
F. Bertaut and F. Forrat, J. Phys. Radium 17 (1956) 129.
S. Geller, Acta Cryst. 10 (1957) 243.
M. Kestigian, J. D. Dickinson and R. Ward, J. Amer. Chem. Soc. 79 (1957) 5598.
H. Brusset, R. Mahe and A. Deboichet, C.R. Acad. Sci. Paris C 274 (1972) 1293.
D. B. Rogers, A. Ferretti, D. H. Ridgley, R. J. Arnott and J. Goodenough, J. Appl. Phys. 37 (1966) 1431.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Guha, J.P., Gaberšček, S. & Kolar, D. Phase equilibrium relations in the V2O3-La2O3 system. J Mater Sci 17, 803–807 (1982). https://doi.org/10.1007/BF00540377
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00540377