Abstract
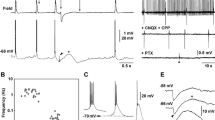
Epileptic discharges were induced by superfusion of rat hippocampal slices with penicillin. Under these conditions the neurons generated paroxysmal depolarization shifts (PDS) after electrical stimulation of Schaffer collaterals. The PDS were followed by large afterhyperpolarizations lasting about 2 s. The mechanisms causing these afterhyperpolarizations were studied in CA1 pyramidal cells. A late component of the after hyperpolarizations, which determined their overall duration, was blocked by intracellular application of EGTA and reduced by superfusion with 8-Br-cAMP. In the same neurons these drugs had a comparable effect on after hyperpolarizations following depolarizing current injections; it was therefore concluded that the late component of the PDS afterhyperpolarizations was caused by a slow Ca2+-activated K+ current. An initial fast component of PDS afterhyperpolarizations, which peaked about 60 ms after PDS onset, was reduced by EGTA but not affected by 8-Br-cAMP suggesting that the fast Ca2+-activated K+ current also contributed to the PDS afterhyperpolarizations. Superfusion of the slice with the γ-aminobutyric acid B receptor (GABAB) antagonists phaclofen or 5-aminovalerate reduced the amplitude of the afterhyperpolarizations during the first 1000 ms but did not affect the late Ca2+-dependent component, indicating that a GABAB-mediated K+ inhibitory postsynaptic potential (IPSP) contributed to the PDS afterhyperpolarization. Intracellular injection of Cl− revealed that an early part of the afterhyperpolarizations lasting about 500 ms was Cl−-dependent. This component was blocked by superfusion of the slices with bicuculline, suggesting that a GABAA-mediated Cl− IPSP contributed to the PDS afterhyperpolarization. The experiments show that different synaptic and intrinsic components with different time courses participate in the generation of PDS afterpotentials.
Similar content being viewed by others
References
Alger BE, Nicoll RA (1980) Epileptiform burst after-hyperpolarization:calcium-dependent potassium potential in hippocampal CA1 pyramidal cells. Science 210:1122–1124
Alger BE, Nicoll RA (19829 Pharmacological evidence for two kinds of GABA receptor on rat hippocampal pyramidal cells studied in vitro. J. Physiol (Lond) 328:125–141
Alger BE, Williamson A (1988) A transient calcium-dependent potassium component of the epileptiform burst afterhyperpolarization in rat hippocampus. J. Physiol (Lond) 399:191–205
Bowery NG, Price GW, Hudson AL, Hill DR, Wilkin GP, Turnbull MJ (1984) GABA receptor multiplicity: visualization of different rceptor types in the mammalian CNS. Neuropharmacology 23:219–231
Davenport J, Schwindt PC, Crill WE (1978) Presynaptic and longlasting postsynaptic inhibition during penicillin-induced spinal seizures. Neurology 28:592–597
Davidoff RA (1983) Studies of neurotransmitter actions (GABA, glycine, and convulsants). In: Ward Jr AA, Penry JK, Purpura D (eds) Epilepsy. Raven, New York, pp 53–85
Dichter MA, Ayala GF (1987) Cellular mechanism of epilepsy: a status report. Science 237:157–164
Dingledine R, Gjerstad L (1979) Penicillin blocks hippocampal IPSPs, unmasking prolonged EPSPs. Brain Res 168:205–209
Domann R, Dorn T, Witte OW (1989a) Giant Cl−-dependent IPSPs following penicillin-induced paroxysmal depolarizations in the hippocampal slice. In: Elsner N. Singer W (eds) Dynamic and plasticity in neuronal systems. Thieme, Stuttgart, p 395
Domann R, Dorn T, Witte OW (1989b) GABAA- and GABAB-dependent IPSPs following penicillin-induced paroxysmal depolarizations in the hippocampal slice. Soc Neurosci Abstr 15:340
Domann R, Dorn T, Witte OW (1989c) Calcium-dependent potassium current following penicillin-induced epileptiform discharges in the hippocampal slice. Exp Brain Res 78:646–648
Dutar P, Nicoll RA (1988) A physiological role for GABAB receptors in the central nervous system. Nature 332:156–158
Haas HL, Schaerer B, Vosmansky H (1979) A simple perfusion chamber for the study of nervous tissue slices in vitro. J Neurosci Methods 1:323–325
Hablitz JJ (1981) Effects of intracellular injections of chloride and EGTA on post epileptiform-burst hyperpolarization in hippocampal neurons. Neurosci Lett 22:159–163
Heinemann U, Konnerth A, Pumain R, Wadman WJ (1986) Extracellular calcium and potassium concentration changes in chronic epileptic brain tissue. In: Delgado-Escueta AV, Ward Jr AA, Woodbury DM, Porter RJ (eds) Adv Neurol 44:641–661
Knowles WD, Schneiderman JH, Wheal HV, Stafstrom CE, Schwartzkroin PA (1984) Hyperpolarizing potentials in guinea pig hippocampal CA3 neurons. Cell Mol Neurobiol 4:207–230
Lancaster B, Adams PR (1986) Calcium-dependent currents generating the afterhyperpolarization of hippocampal neurons. J Neurophysiol 55:1268–1282
Lancaster B, Nicoll RA (1987) Properties of two calcium-activated hyperpolarizations in rat hippocampal neurons. J. Physiol (Lond) 389:187–203
Macdonald RL, Barker JL (1978) Specific antagonism of GAB-Amediated postsynaptic inhibition in cultured mammalian spinal cord neurons: a common mode of convulsant action. Neurology 28:325–330
Madison BV, Nicoll RA (1986) Cyclic adenosine 3′,5′-monophosphate mediates beta-receptor actions of noradrenalin in rat hippocampal cells. J Physiol (Lond) 372:245–259
Matsumoto H, Ajmone-Marsan C (1964a) Cortical cellular phenomena in experimental epilepsy: interictal manifestations. Exp Neurol 9:286–305
Matsumoto H, Ajmone-Marsan C (1964b) Cortical cellular phenomena in experimental epilepsy: ictal manifestations. Exp Neurol 9:305–326
McCormick DA (1989) GABA as an inhibitory neurotransmitter in human cerebral cortex. J Neurophysiol 62:1018–1027
McCormick DA, Connors BW, Lighthall JW, Prince DA (1985) Comparative electrophysiology of pyramidal and sparsely spiny stellate neurons of the neocortex. J Neurophysiol 54:782–806
Nakahiro M, Saito K, Yamada I, Yoshida H (1985) Antagonistic effect of delta-aminovaleric acid on bicuculline-insensitive gamma-aminobutyric acidB (GABAB) sites in the rat's brain. Neurosci Lett 57:263–266
Newberry NR, Nicoll RA (1984) A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol (Lond) 348:239–254
Prince DA (1968) Inhibition in ‘epileptic’ neurons. Exp Neurol 21:307–321
Schwartzkroin PA, Stafstrom CE (1980) Effects of EGTA on the calcium-activated afterhyperpolarization in hippocampal CA3 pyramidal cells. Science 210:1125–1126
Segal M, Barker JL (1984) Rat hippocampal neurons in culture: properties of GABA-activated Cl− ion conductance. J Neurophysiol 51:500–515
Storm JF (1987) Action potential repolarization and a fast afterhyperpolarization in rat hippocampal pyramidal cells. J Physiol (Lond) 385:733–759
Thalmann RH, Ayala GF (1982) A late increase in potassium conductance follows synaptic stimulation of granule neurons of the dentate gyrus. Neurosci Lett 29:243–248
Traub RD, Miles R, Wong RKS (1987) Models of synchronized hippocampal bursts in the presence of inhibition: I. Single population events. J Neurophysiol 58:739–751
Valle E, Witte OW (1988) Glial cell depolarization during penicill-ininduced interictal epileptic discharges in the motor cortex of the rat in vivo. Pflügers Arch 411 [Suppl 1]: R119
Weiss DS, Hablitz JJ (1984) Interaction of penicillin and pentobarbital with inhibitory synaptic mechanisms in neocortex. Cell Mol Neurobiol 4:301–317
Witte OW, Speckmann E-J, Walden J (1987) Motorcortical epileptic foci in vivo: actions of a calcium channel blocker on paroxysmal neuronal depolarizations. Electroencephalogr Clin Neurophysiol 66:43–55
Witte OW, Uhlig S, Valle E (1988) Potassium-dependent slow afterpotentials of penicillin-induced neuronal paroxysmal depolarization shifts in the motor cortex of the rat. Pflügers Arch 412 [Suppl 1]:R68
Witte OW, Uhlig S, Valle E (1989) Separation of different types of afterpotentials following penicillin-induced paroxysmal depolarization shifts in neurons of the motor cortex of the rat. Neurosci Lett 101:51–56
Wong RKS, Prince RD (1978) Participation of calcium spikes during intrinsic burst firing in hippocampal neurons. Brain Res 159:385–390
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Domann, R., Dorn, T. & Witte, O.W. Afterpotentials following penicillin-induced paroxysmal depolarizations in rat hippocampal CA1 pyramidal cells in vitro. Pflugers Arch. 417, 469–478 (1991). https://doi.org/10.1007/BF00370941
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00370941