Abstract
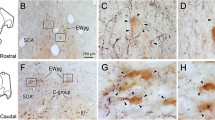
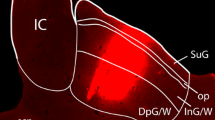
The retinal innervation, cytoarchitectural, and immunohistochemical organization of the suprachiasmatic nucleus (SCN) was studied in the domestic sheep. The SCN is a large elongated nucleus extending rostrocaudally for roughly 3 mm in the hypothalamus. The morphology is unusual in that the rostral part of the nucleus extends out of the main mass of the hypothalamus onto the dorsal aspect of the optic chiasm. Following intraocular injection of wheat-germ agglutininhorseradish peroxidase or tritiated amino acids, anterograde label is distributed throughout the SCN. Retinal innervation of the SCN is bilaterally symmetric or predominantly ipsilateral. Quantitative image analysis demonstrates that, although the amount of autoradiographic label is greatest in the ventral and central parts of the nucleus, density varies progressively between different regions. In addition to the SCN, retinal fibers are also seen in the medial preoptic area, the anterior and lateral hypothalamic areas, the dorsomedial hypothalamus, the retrochiasmatic area, and the basal telencephalon. Whereas the SCN can be identified using several techniques, complete delineation of the nucleus requires combined tract tracing, cytoarchitectural, and histochemical criteria. Compared with the surrounding hypothalamic regions, the SCN contains smaller, more densely packed neurons, and is largely devoid of myelinated fibers. Cell soma sizes are smaller in the ventral SCN than in the dorsal or lateral parts, but an obvious regional transition is lacking. Using Nissl, myelin, acetylcholinesterase, and cytochrome oxidase staining, the SCN can be clearly distinguished in the rostral and medial regions, but is less differentiated toward the caudal pole. Immunohistochemical demonstration of several neuropeptides shows that the neurochemical organization of the sheep SCN is heterogeneous, but that it lacks a distinct compartmental organization. Populations of different neuropeptide-containing cells are found throughout the nucleus, although perikarya positive for vasoactive intestinal polypeptide and fibers labeled for methionine-enkephalin are predominant ventrally; neurophysine-immunoreactive cells are more prominent in the dorsal region and toward the caudal pole. The results suggest that the intrinsic organization of the sheep SCN is characterized by gradual regional transitions between different zones.
Similar content being viewed by others
References
Argawala S, Petry HM, May III JG (1989) Retinal projections in the ground squirrel (Citellus tridecemlineatus). Vis Neurosci 3:537–549
Balkema GW, Dräger UC (1990) Origins of uncrossed retinofugal projections in normal and hypopigmented mice. Vis Neurosci 4:593–604
Bittman IL, Crandell RG, Lehman MN (1989) Influences of the paraventricular and suprachiasmatic nuclei and olfactory bulbs on melatonin responses in the golden hamster. Biol Reprod 40:118–126
Bosler O, Beaudet A (1985) VIP neurons as prime synaptic targets for serotonin afferents in rat suprachiasmatic nucleus: a combined radioautographic and immunocytochemical study. J Neurocytol 14:749–763
Card JP, Moore RY (1982) Ventral lateral geniculate nucleus neurons efferent to the rat suprachiasmatic nucleus exhibit avian pancreatic polypeptide-like immunoreactivity. J Comp Neurol 206:390–396
Card JP, Moore RW (1989) Organization of lateral geniculate-hypothalamic connections in the rat. J Comp Neurol 284:135–147
Cassone VM (1990) Effects of melatonin on vertebrate circadian systems. Trends Neurosci 13:457–464
Cassone VM, Speh JC, Card JP, Moore RY (1988) Comparative anatomy of the mammalian hypothalamic suprachiasmatic nucleus. J Biol Rhythms 3:71–91
Chemineau P, Malpaux B, Delgadillo JA, Guérin Y, Ravault JP, Thimonier J, Pelletier J (1992) Control of sheep and goat reproduction: use of light and melatonin. Animal Reprod Sci 30:157–184
Cooper HM, Mick G, Magnin M (1978) Retinohypothalamic pathway revisited in mammals: an unusual ipsilateral asymmetry in primates. Soc Neurosci Abstr 13:863
Cooper HM, Mick G, Magnin M (1989) Retinal projection to mammalian telencephalon. Brain Res 477:350–357
Cooper HM, Herbin M, Nevo E (1993a) The visual system of a naturally microphthalmic mammal: the blind mole rat, Spalax ehrenberghi. J Comp Neurol 328:313–350
Cooper HM, Herbin M, Nevo E (1993b) Ocular regression conceals adaptive progression of the visual system in a blind subterranean mammal. Nature 361:156–159
Cooper HM, Tessonneaud A, Caldani A, Locatelli A, Richard S, Viguier-Martinez M-C (1993c) Morphology and distribution of retinal ganglion cells (RGC) projecting to the suprachiasmatic nucleus in the sheep. Am Soc Neurosci Abstr 19:1704
Cooper HM, Parvopassu F, Herbin M, Magnin M (1994) Neuroanatomical pathways linking vision and olfaction in mammals. Neuroendocrinology (in press)
Cusick CG, Kaas JH (1982) Retinal projections in adult and newborn grey squirrels. Dev Brain Res 4:275–284
De Olmos J, Hardy H, Heimer L (1978) The afferent connections of the main and the accessory olfactory bulb formations in the rat: an experimental HRP-study. J Comp Neurol 181:213–244
Diepen RB (1941) The hypothalamic nuclei and their ontogenetic development in domestic ungulates (Ovis aries). Unpublished thesis, Amsterdam, p 97
Dubois MP, Barry J (1974) Répartition comparée de trois neurofacteurs hypothalamiques: LHRF, SFIF et neurophysine dans l'hypothalamus et l'éminence médiane: étude en immunofluorescence. Ann Endocrinol (Paris) 35:663–664
Earnest DJ, Sladek CD (1986) Circadian rhythms of vasopressin release from individual rat suprachiasmatic explants in vitro. Brain Res 382:129–133
Finley JCW, Maderdrut JL, Petrusz P (1981) The immunocytochemical localization of enkephalin in the central nervous system of the rat. J Comp Neurol 198:541–565
Fletcher KL, Terman M, Silverman AJ, Stark RI (1990) Development of the retino-hypothalamic tract (RHT) in fetal sheep. Soc Neurosci Abstr 16:601
Follénius E, Dubois MP (1979) Differentiation immunocytologique de l'innervation hypophysaire de la carpe à l'aide de sérume anti-mét-enkephaline et anti-B-endorphine. CR Acad Sci Paris 288:639–644
Fuller CA, Murikami DM, Miller DA (1984) Cell projections and cytochrome oxidase activity within the hypothalamus of the rat and cat. Soc Neurosci Abstr 10:503
Gallyas F (1979) Silver staining of myelin by means of physical development. Neurol Res 1:203–209
Gibson AR, Hansma DI, Houk JC, Robinson FR (1984) A sensitive low artifact TMB procedure for the demonstration of WGA-HRP in the CNS. Brain Res 298:235–241
Gillette MU (1986) The suprachiasmatic nuclei: circadian phase shifts induced at time of hypothalamic slice preparation are conserved in vitro. Brain Res 379:176–181
Gillette MU, Reppert SM (1987) The hypothalamic suprachiasmatic nuclei: circadian patterns of vasopressin secretion and neuronal activity in vitro. Brain Res Bull 19:135–139
Green DJ, Gillette R (1982) Circadian rhythm of firing rate recorded from single cells in the rat suprachiasmatic nucleus brain slice. Brain Res 245:198–200
Groos GA, Meijer JH (1985) Effects of illumination on suprachiasmatic nucleus electrical discharge. Ann NY Acad Sci, 453:134–146
Güldner F-H (1976) Synaptology of the rat suprachiasmatic nucleus. Cell Tissue Res 165:509–544
Hardy HL, Heimer L, Switzer R, Watkins D (1976) Simultaneous demonstration of horseradish peroxidase and acetylcholinesterase. Neurosci Lett 3:1–5
Hendrickson AE, Wagoner N, Cowan WN (1972) An autoradiographic and electron microscopic study of retinohypothalamic projections. Z Zellforsch 135:1–26
Ichikawa T, Hirata Y (1986) Organization of choline acetlytransferase-containing structures in the forebrain of the rat. J Neurosci 6:281–292
Inagaki S, Parent A (1985) Distribution of enkephalin-immunoreactive neurons in the forebrain and upper brainstem of the squirrel monkey. Brain Res 359:267–280
Inouye SIT, Kawamura H (1979) Persistence of circadian rhythmicity in a mammalian hypothalamic “island” containing the surpachiasmatic nucleus. Proc Natl Acad Sci USA 76:5962–5966
Inouye SIT, Kawamura H (1982) Characteristics of a circadian pacemaker in the suprachiasmatic nucleus. J Comp Physiol 146:153–166
Johnson RF, Morin LP, Moore RY (1988) Retinohypothalamic projections in hamster and rat demonstrated using cholera toxin. Brain Res 462:301–312
Karsch FJ, Robinson JE, Woodfill CJI, Brown MB (1989) Circannual cycles of luteinizing hormone and prolactin secretion in ewes during prolonged exposure to a fixed photoperiod: evidence for an endogenous reproductive rhythm. Biol Reprod 41:1034–1046
Kawata M, Ueda S, Yamashita H, Sano Y (1987) Immunohistochemical studies on the distribution of neuropeptides and serotonin in the Brattelboro rat. Arch Histol Jpn 50:1–14
Kudo M, Yamamoto M, Nakamura Y (1991) Suprachiasmatic nucleus and retinohypothalamic projections in moles. Brain Behav Evol 38:332–338
Künzle, H (1988) Retinofugal projections in hedgehog-tenrecs (Echinops telfairi and Setifer setosus). Anat Embryol 178:77–93
Le Gros Clark WE (1936) The topography and homologies of the hypothalamic nuclei in man. J Anat 70:203–214
Legan SJ, Winans WW (1981) The photoneuroendocrine control of seasonal breeding in the ewe. Gen Comp Endocrinol 45:317–328
Lehman MN, Silver R, Gladstone WR, Kahn RM, Gibson M, Bittman EL (1987) Circadian rhythmicity restored by neural transplant. Immunocytochemical characteristics with the host brain. J Neurosci 7:1626–1638
Lenn NJ, Beebe B, Moore RY (1977) Postnatal development of the suprachiasmatic hypothalamic nucleus of the rat. Cell Tissue Res 178:463–475
Levine JD, Weiss ML, Rosenwasser AM, Miselis RR (1991) Retinohypothalamic tract in the female albino rat: a study using horseradish peroxidase conjugated to cholera toxin. J Comp Neurol 306:344–360
Lydic RL, Albers HE, Tepper B, Moore-Ede MC (1982) Three dimensional structure of the mammalian suprachiasmatic nuclei: a comparative study of five species. J Comp Neurol 204:225–237
Magnin M, Cooper HM, Mick G (1989) Retinohypothalamic pathway: a breach in the law of Newton-Müller-Gudden? Brain Res 488:390–397
Mai JK, Kedziora O, Teckhaus L, Sofroniew MV (1991) Evidence for subdivisions in the human suprachiasmatic nucleus. J Comp Neurol 305:508–525
Martinet L, Servière J, Peytevin J (1992) Direct projections of the “non-image forming” system to the hypothalamus, anterodorsal thalamus and basal telencephalon of mink. Exp Brain Res 89:373–382
Meijer JH, Rietveld WJ (1989) Neurophysiology of the suprachiasmatic circadian pacemaker in rodents. Physiol Rev 69:671–707
Meijer JH, Groos JA, Rusak B (1986) Luminance coding in a circadian pacemaker: the suprachiasmatic nucleus of the rat and hamster. Brain Res 382:109–118
Meijer JM, Rusak B, Harrington ME (1989) Photically responsive neurons in the hypothalamus of a diurnal ground squirrel. Brain Res 501:315–323
Mesulam MM (1978) Tetramethyl benzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Mick M, Cooper HM, Magnin M (1993) Retinal projection to the olfactory tubercle and basal telencephalon in primates. J Comp Neurol 327:295–219
Mikkelsen JD (1990a) A neuronal projection from the lateral geniculate nucleus to the lateral hypothalamus of the rat demonstrated with PHA-L tracing. Neurosci Lett 116:58–63
Mikkelsen JD (1990b) Projections from the lateral geniculate nucleus to the hypothalamus of the Mongolian gerbil (Meriones unguiculatus): an anterograde and retrograde tracing study. J Comp Neurol 299:493–508
Mizuno N, Sumi M-U, Tashiro T, Takahashi O, Satoda T (1991) Retinofugal projections in the house musk shrew, Suncus murinus. Neurosci Lett 125:133–135
Møller M, Mikkelsen JD, Fahrenkrug J, Korf H-W (1985) The presence of vasoactive intestinal polypeptide (VIP)-like immunoreactive nerve fibers and VIP receptors in the pineal gland of the Mongolian gerbil. J Comp Neurol 273:87–98
Moore RY (1973) Retinohypothalamic projections in mammals. A comparative study. Brain Res 49:403–409
Moore RY (1983) Organization and function of a central nervous system circadian oscillator: the suprachiasmatic hypothalamic nucleus. Fed Proc Am Soc Exp Biol 42:2783–2789
Moore RY, Card JP (1990) Neuropeptide-Y in the circadian timing system. Ann NY Acad Sci 611:247–257
Moore RY, Lenn NJ (1972) A retinohypothalamic projection in the rat. J Comp Neurol 146:1–14
Morin LP, Blanchard J, Moore RY (1992) Intergeniculate leaflet and suprachiasmatic nucleus organization and connections in the golden hamster. Vis Neurosci 8:219–230
Murikami DM, Fuller CA (1988) The postnatal development of oxidative metabolism in the suprachiasmatic nucleus of the rat. Neurosci 24:977–986
Murikami DM, Fuller CA (1990) The retinohypothalamic projection and oxidative metabolism in the suprachiasmatic nucleus of primates and tree shrews. Brain Behav Evol 35:302–312
Murikami DM, Miller JD, Fuller CA (1989) The retinohypothalamic tract in the cat: retinal ganglion cell morphology and pattern of projection. Brain Res 482:283–296
Murikami N, Takahashi K, Kawashiwa K (1984) Effect of light on the acetylcholine concentrations of the suprachiasmatic nucleus in the rat. Brain Res 311:358–360
Nelson DE, Takahashi JS (1991) Sensitivity and integration in a visual pathway for circadian entrainment in the hamster (Mesocricetus auratus). J Physiol 439:115–145
Nelson RJ, Zucker I (1981) Photoperiodic control of reproduction in olfactory-bulbectomized rats. Neuroendocrinology 32:266–271
Ortavant R, Bocquier F, Pelletier J, Ravault JP, Thimonier J, Volland-Nail P (1988) Scasonality and reproduction in sheep and its control by photoperiod. Aust J Biol Sci 41:69–85
Pickard GE (1980) Morphological characteristics of retinal ganglion cells projecting to the suprachiasmatic nucleus: a horseradish peroxidase study. Brain Res 183:458–465
Pickard GE (1982) The afferent connections of the suprachiasmatic nucleus of the golden hamster with emphasis on the retinohypothalamic projection. J Comp Neurol 211:65–83
Pickard GE (1985) Bifurcating axons of retinal ganglion cells terminate in the hypothalamic suprachiasmatic nucleus and the intergeniculate leaflet of the thalamus. Neurosci Lett 55:211–217
Pickard GE, Friauf E (1990) Morphological features of Lucifer yellow (LY) filled retinal ganglion cells innervating the suprachiasmatic nucleus (SCN). Soc Neurosci Abstr 1:602
Pickard GE, Silverman A-J (1981) Direct retinal projections to the hypothalamus, piriform cortex, and accessory optic nuclei in the golden hamster as demonstrated by a sensitive anterograde horseradish peroxidase technique. J Comp Neurol 196:155–172
Pieper DR, Tang Y-K, Lipski TP, Subramanian MG, Newman SW (1984) Olfactory bulbectomy prevents the gonadal regression associated with short photoperiod in male golden hamsters. Brain Res 321:183–186
Pieper DR, Unthank PD, Shuttie DA, Lobocki CA, Swann JM, Newman S, Subramanian MG (1987) Olfactory bulbs influence testosterone feedback on gonadotropin secretion in male hamsters on long or short photoperiod. Neuroendocrinology 46:318–323
Pieper DR, Newman SW, Lobocki CA, Gogola G (1989) Bilateral transection of the lateral olfactory tract but not removal of the vomeronasal organs inhibits short-photoperiod induced testicular regression in golden hamsters. Brain Res 485:382–390
Pol AN van den (1980) The hypothalamic suprachiasmatic nucleus of the rat: intrinsic anatomy. J Comp Neurol 191:661–702
Pol AN van den, Tsujimoto KL (1985) Neurotransmitters of the hypothalamic suprachiasmatic nucleus: immunocytochemical analysis of 25 neuronal antigens. Neuroscience 15:1049–1086
Pol AN van den, Finkbeiner SM, Cornell-Bell AH (1992) Calcium excitability and oscillations in suprachiasmatic nucleus neurons and glia in vitro. J Neurosci 12:2648–2664
Possidente B, Lumia AR, McGinnis MY, Teicher MH, deLomos E, Sterner L, Deros L (1990) Olfactory bulb control of circadian activity rhythm in mice. Brain Res 513:325–328
Purves D, Rubin E, Snider WD, Lichtman J (1986) Relation of animal size to convergence, divergence, and neuronal number in peripheral sympathetic pathways. J Neurosci 6:158–163
Ralph MR, Lehman MN (1991) Transplantation — a new tool in the analysis of the mammalian hypothalamic circadian pacemaker. Trends Neurosci 14:362–366
Ralph MR, Foster RG, Davis FC, Menaker M (1990) Transplanted suprachiasmatic nucleus determines circadian period. Science 247:975–978
Rao ZR, Yamano M, Wanaka A, Tatehata T, Shiosaka S, Tohyama M (1987) Distribution of cholinergic neurons and fibers in the hypothalamus of the rat using choline acetyltransferase as a marker. Neuroscience 20:923–934
Rusak B (1977) The role of the suprachiasmatic nuclei in the generation of circadian rhythms in the golden hamster Mesocricetus auratus. J Comp Physiol 118:145–164
Rusak B (1989) The mammalian circadian system. Models and physiology. J Biol Rhythms 4:121–134
Rusak B, Bina KG (1990) Neurotransmitters in the mammalian circadian system. Annu Rev Neurosci 13:387–401
Sato T, Kawamura H (1984) Circadian rhythms in multiple unit activity inside and outside the suprachiasmatic nucleus in the diurnal chipmunk (Eutaminus sibiricus). Neurosci Res 1:45–52
Scalia F, Winans SS (1976) The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol 161:31–56
Schmued LC, Swanson LW, Sawchenko PE (1982) Some fluorescent counterstains for neuroanatomical studies. J Histochem Cytochem 30:123–128
Shibata S, Moore RY (1988) Electrical and metabolic activity of suprachiasmatic nucleus neurons in hamster hypothalamic slices. Brain Res 438:374–378
Skeen LC, Hall WC (1977) Efferent projections of the main and accessory olfactory bulb in the tree shrew. J Comp Neurol 172:1–36
Smale L, Blanchard J, Moore RY, Morin LP (1991) Immunocytochemical characterization of the suprachiasmatic nucleus and the intergeniculate leaflet in the diurnal ground squirrel, Spermophilus lateralis. Brain Res 563:77–86
Steinbush HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat. Cell bodies and terminals. Neuroscience 6:557–618
Stephan FK, Zucker I (1972) Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA 69:1583–1586
Swaab DF, Hofman DA (1990) An enlarged suprachiasmatic nucleus in homosexual men. Brain Res 537:141–148
Swaab DF, Fliers E, Partiman TS (1985) The suprachiasmatic nucleus of the human brain in relation to sex, age and human dementia. Brain Res 342:37–44
Tago H, McGreer PL, Bruce G, Hersh LB (1987) Distribution of choline acetyltransferase-containing neurons of the hypothalamus. Brain Res 415:49–62
Takahashi JS, Zatz M (1982) Regulation of circadian rhythmicity. Science 217:1104–1111
Torrealba F, Parraguez, VH, Reyes G, Valenzuela G, Seron-Ferré M (1993) Prenatal development of the retinohypothlamic pathway and the suprachiasmatic nucleus in the sheep. J Comp Neurol 338:304–316
Tigges J, Bos J, Tigges M (1979) An autoradiographic investigation of the subcortical visual system in chimpanzee. J Comp Neurol 172:367–380
Tillet Y, Caldani M, Tramu G (1989) Immunohistochemical characteristics of the sheep suprachiasmatic nucleus. J Chem Neuroanat 2:215–226
Turek FW (1985) Circadian neural rhythms in mammals. Annu Rev Physiol 47:49–64
Watts AG, Swanson LW (1987) Efferent projections of the suprachiasmatic nucleus. II. Studies using retrograde transport of fluorescent dyes and simultaneous peptide immunohistochemistry in the rat. J Comp Neurol 258:230–252
Watts AG, Swanson LW, Sanchez-Watts G (1987) Efferent projections of the suprachiasmatic nucleus. I. Studies using anterograde transport of Phaseolus vulgarus leucoagglutinin in the rat. J Comp Neurol 258:204–229
Wong-Riley M (1979) Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res 171:11–28
Woodfill CGI, Robinson JI, Malpaux B, Karsch FJ (1991) Synchronization of the circadian reproductive rhythm of the ewe by discrete photoperiodic signals. Biol Reprod 45:110–121
Youngstrom TG, Weiss ML, Nunez AA (1991) Retinofugal projections to the hypothalamus, anterior thalamus and basal forebrain in hamsters. Brain Res Bull 26:403–411
Zamboni L, Martino L de (1967) Buffered picric acid formaldehyde: a new rapid fixative for electron microscopy. J Cell Biol 35:148A
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tessonneaud, A., Cooper, H.M., Caldani, M. et al. The suprachiasmatic nucleus in the sheep: retinal projections and cytoarchitectural organization. Cell Tissue Res 278, 65–84 (1994). https://doi.org/10.1007/BF00305779
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00305779