Abstract
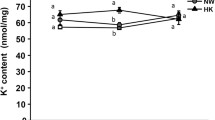
Mitochondria-rich cells (MRC) of the amphibian epidermis are responsible for active chloride uptake at low external salinity, and new MRCs are recruited in response to exposure to distilled (deionized) water. The time-course of this recruitment, the tissue kinetics and ion transport have been studied in toads (Bufo bufo) immediately before, and after 2,7, and 14 days exposure to distilled water. General epidermal structure was not affected. However, the numbers of MRCs per mm2 (DMRC) increased throughout the experiment as revealed by staining of epidermal sheets with AgNO3 (Ag) or methylene blue (MB). Part of the increased DMRC was accounted for by an increase in MRC subpopulation(s) that stained neither with Ag nor MB. The cell birth rate (Kb) decreased and cell loss by moulting (Kd) increased without any significant change in epidermal cell pool size, indicating a reduced apoptotic rate. The increase in DMRC was accompanied by a 3-fold increase in Cl- current (ICl). At day-2 there was a transient reduction in the ICl per MRC. H+ secretion was progressively reduced during prolonged exposure to distilled water. Thus, at day-2 MRCs appeared incompletely differentiated as indicated by decreased ICl and H+ flux per MRC, and by the increased proportion of MRCs unstained by Ag or MB. Full Cl- (but not H+) transport capacity, was restored at day-7. We conclude that increased DMRC following exposure to low external Cl-, rather than being due to an increased Kb, is the combined effect of a decreased apoptotic rate and an increased rate of differentiation, where ‘morphological differentiation’ precedes ‘functional differentiation’.
Similar content being viewed by others
References
Balls M, Brown D, Flemming N (1976) Long-term amphibian organ culture. In: Prescott DM (ed) Methods in Cell Biology, vol 13. Academic Press, New York San Francisco London, pp 213–238
Bendsen J (1956) Shedding of the skin in the common toad Bufo bufo. Vidsk Medd Dansk Naturhist Foren 118:211–225
Budtz PE (1979) Epidermal structure and dynamics of the toad, Bufo bufo, deprived of the pars distalis of the pituitary gland. J Zool (Lond) 189:57–92
Budtz PE (1985a) Epidermal tissue homeostatis. I. Cell pool size, cell birth rate and cell loss by moulting in the intact toad, Bufo bufo. Cell Tissue Kinet 18:521–532
Budtz PE (1985b) Epidermal tissue homeostasis. II. Cell pool size, cell birth rate and cell loss in toads deprived of the pars distalis of the pituitary gland. Cell Tissue Kinet 18:533–542
Budtz PE (1986) Amphibian skin as a model in studies on epidermal homeostasis. In: Marks R, Plewig B (eds) Skin Models. Springer, Berlin Heidelberg New York, pp 58–72
Budtz PE (1988) Epidermal tissue homeostasis. III. Effect of hydrocortisone on cell pool size, cell birth rate and cell loss in normal toads and in toads deprived of the pars distalis of the pituitary gland. Cell Tissue Kinet 21:259–270
Budtz PE (1991) Tissue kinetic homeostasis: Studies on toad epidermis. August Krogh Publications, Copenhagen, 1:3–94
Budtz PE, Larsen LO (1973) Structure of the toad epidermis during the moulting cycle. I. Light microscopic observations in Bufo bufo. Z Zellforsch 144:353–365
Budtz PE, Spies I (1989) Epidermal tissue homeostasis. Apoptosis and cell emigration as mechanisms of controlled cell deletion in the epidermis of the toad, Bufo bufo. Cell Tissue Res 256:475–486
Budtz PE, Christoffersen BC, Johansen JS, Spies I, Willumsen NJ (1993) Kinetics of toad epidermal mitochondria-rich cells (MRC). Cell Prolif 26:467
Carasso N, Favard P, Jard S, Rajerison RM (1971) The isolated frog skin epithelium. I. Preparation and general structure in different physiological states. J Microscopie 10:315–330
Devuyst O, Beaujean V, Crabbé J (1991) Effects of environmental conditions on mitochondria-rich cell density and chloride transport in toad skin. Pflügers Arch 417:577–581
Ehrenfeld J, Masoni A, Garcia-Romeu F (1976) Mitochondria-rich cells of frog skin in transport mechanisms: morphological and kinetic studies on transepithelial excretion of methylene blue. Am J Physiol 231:120–126
Fox H (1986) The skin of Amphibia: Epidermis. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the integument, vol 2, Vertebrates. Springer, Berlin Heidelberg New York, pp 472–498
Harvey BJ (1992) Energization of sodium absorption by the H+-ATPase pump in mitochondria-rich cells of frog skin. J Exp Biol 172:289–309
Holmes W (1968) Empiricism — Silver methods and the nerve axon. In: McGee-Russell SM, Ross KFA (eds) Cell structure and its interpretation. Edward Arnold, London, pp 95–102
Ilic V, Brown D (1980) Modification of mitochondria-rich cells in different ionic conditions: changes in cell morphology and cell number in the skin of Xenopus laevis. Anat Rec 196:153–161
Katz U (1986) The role of amphibian epidermis in osmoregulation and its adaptive response to changing environment. In: Bereiter-Hahn J, Matoltsy AG, Richards KS (eds) Biology of the Integument, vol 2, Vertebrates. Springer, Berlin Heidelberg New York, pp 472–498
Katz U, Gabbay S (1988) Mitochondria-rich cells and carbonic anhydrase content of toad skin epithelium. Cell Tissue Res 251:425–431
Katz U, Gabbay S (1993) Band 3 protein and ion transfer across epithelia: mitochondria-rich cells in the amphibian skin epithelium. In: Scheid P (ed) Respiration in health and disease. Gustav Fischer, Stuttgart Jena New York, pp 75–82
Kristensen P, Ussing HH (1992) Epithelial organization. In: Seldin DW, Giebisch G (eds) The Kidney: Physiology and pathophysiology, 2nd edn. Raven Press, New York, pp 265–285
Krogh A (1937) Osmotic regulation in the frog (Rana esculenta) by active absorption of chloride ions. Skand Arch Physiol 76:60–74
Lane EB, Bártek J, Purkis PE, Leigh IR (1985) Keratinocytes in differentiating skin. Ann NY Acad Sci 455:241–258
Larsen EH (1988) NaCl transport in amphibian skin. In: Greger R (ed) Advances in Comparative and Environmental Physiology. Springer, Berlin Heidelberg, pp 189–248
Larsen EH (1991) Chloride transport by a high resistance heterocellular epithelium. Physiol Rev 71:235–283
Larsen EH, Willumsen NJ, Christoffersen BC (1992) Role of proton pump of mitochondria-rich cells for active transport of chloride ions in toad skin epithelium. J Physiol 450:203–216
Larsen LO (1976) Physiology of moulting. In: Lofts B (ed) Physiology of Amphibians, vol III. Academic Press, New York San Francisco London, pp 53–100
Levi H, Nielsen A (1982) An autoradiographic study of cell kinetics in epidermis of the toad Bufo bufo bufo (L.). J Invest Dermatol 79:292–296
Machen T, Ehrlich D (1975) Some features of hydrogen (ion) secretion by the frog skin. Biochem Biophys Acta 406:120–130
Page RD, Mia AJ, Buttar S, Yorio T (1990) Adaptive changes of H+ secreting cells in the epidermis of the leopard frog Rana pipiens. Comp Biochem Physiol [C] 96:245–251
Ussing HH, Zerahn K (1951) Active sodium transport as the source of electric current in the short-circuited isolated frog skin. Acta physiol Scand 23:110–127
Willumsen NJ, Larsen EH (1985) Passive Cl- currents in toad skin: potential dependence and relation to mitochondria-rich cell density. In: Gilles R, Gilles-Baillien M (eds) Transport processes, iono- and osmoregulation. Springer, Berlin Heidelberg, pp 20–30
Willumsen NJ, Larsen EH (1986) Membrane potentials and intracellular Cl- activity of toad skin epithelium in relation to activation and deactivation of the transepithelial Cl- conductance. J Membrane Biol 94:173–190
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Budtz, P.E., Christoffersen, B.C., Johansen, J.S. et al. Tissue kinetics, ion transport, and recruitment of mitochondria-rich cells in the skin of the toad (Bufo bufo) in response to exposure to distilled water. Cell Tissue Res 280, 65–75 (1995). https://doi.org/10.1007/BF00304512
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00304512