Abstract
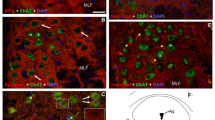
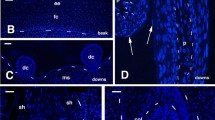
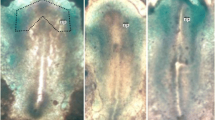
In bird skin, nerve fibres develop in the dermis but do not enter the epidermis. In co-cultures of 7-day-old chick embryo dorsal root ganglia and epidermis, the neurites also avoid the epidermis. Previous studies have shown that chondroitin sulphate proteoglycans may be involved. Chondroitin sulphate has therefore been visualized by immunocytochemistry, using themonoclonal antibody CS-56, both in vivo and in vitro using light and electron microscopy. Its distribution was compared to those of 2 other chondroitin sulphate epitopes and to that of the growing nerve fibres. In cultures of epidermis from 7-day-old embryonic chicks, immunoreactivity is found uniformly around the epidermal cells while at 7.5 days the distribution in dermis is heterogeneous, and particularly marked in feather buds. In vivo, chondroitin sulphate immunoreactivity is detected in the epidermis, on the basal lamina, on the surfaces of fibroblasts and along collagen fibrils. This localization is complementary to the distribution of cutaneous nerves. Chondroitin sulphate in the basal lamina could prevent innervation of the epidermis and the dermal heterogeneities could partly explain the nerve fibres surrounding the base of the feathers. Chondroitin sulphate could therefore be important for neural guidance in developing chick skin.
Similar content being viewed by others
References
Avnur Z, Geiger B (1984) Immunocytochemical localization of native chondroitin sulfate in tissues and cultured cells using specific monoclonal antibody. Cell 38:811–822
Bray D, Hollenbeck PJ (1988) Growth cone motility and guidance. Ann Rev Cell Biol 4:43–61
Brittis PA, Canning DR, Silver J (1992) Chondroitin sulfate as a regulator of neuronal patterning in the retina. Science 255:733–736
Bronner-Fraser M, Stern C (1991) Effects of mesodermal tissues on avian neural crest cell migration. Dev Biol 143:213–217
Carbonetto S, Gruver MM, Turner DC (1983) Nerve fibre growth in culture on fibronectin, collagen and glycosaminoglycan substrates. J Neurosci 3:2324–2335
Ciment G, Weston JA (1982) Early appearance in neural crest and crest-derived cells of an antigenic determinant present in avian neurones. Dev Biol 93:355–367
Ciment G, Ressler A, Letourneau PC, Weston JA (1986) A novel intermediate-filament associated protein, NAPA-73, that binds to different filament types at different stages of nervous system development. J Cell Biol 102:246–251
Davies JA, Cook GMW (1991) Growth cone inhibition-an important mechanism in neural development. BioEssays 13:11–15
Davies JA, Cook GMW, Stern CD, Keynes RJ (1990) Isolation from chick somites of a glycoprotein that causes collapse of dorsal root ganglion growth cones. Neuron 2:11–20
Dodd J, Jessel TM (1991) Axon guidance and the patterning of neuronal projections in vertebrates. Science 242:692–699
Evangelisti R, Bodo M, Caruso A, Becchetti E, Carinci P (1984) Extracellular glycosaminoglycans (GAG) released by chick embryonic fibroblasts A possible involvement of surface receptors. Cell Tissue Res 238:241–245
Fernandez MS, Dennis JE, Drushel RF, Carrino DA, Kimata K, Yamagata M, Caplan AI (1991) The dynamics of compartmentalization of embryonic muscle by extracellular matrix molecules. Dev Biol 147:46–61
Fichard A, Verna JM, Saxod R (1990) Effects of tunicamycin on the avoidance reaction of epidermis by sensory neurites in co-cultures. Int J Dev Neurosci 8:245–254
Fichard A, Verna JM, Olivares J, Saxod R (1991) Involvement of a chondroitin sulfate proteoglycan in the avoidance of chick epidermis by dorsal root ganglia fibers: a study using β-D-xyloside. Dev Biol 148:1–9
Hammerback JA, Letourneau PC (1986) Neurite extension across regions of low substratum affinity: implications for the guidepost hypothesis of axonal pathfinding. Dev Biol 117:655–662
Hedman K, Johanssen S, Vartio T, Kjellen L, Vaheri A, Hook M (1982) Structure of the pericellular matrix: association of heparan and chondroitin sulfates with fibronectin-procollagen fibres. Cell 28:663–671
Hoffman S, Crossin KL, Edelman GM (1988) Molecular forms, binding functions, and developmental expression patterns of cytotactin and cytotactin-binding proteoglycan, an interactive pair of extracellular matrix molecules. J Cell Biol 106:519–532
Jahoda CAB, Mauger A, Sengel P (1987) Histochemical localization of skin glycosaminoglycans during feather development in the chick embryo. Roux's Arch Dev Biol 196:303–315
Jiang TX, Chuong CM (1992) Mechanism of skin morphogenesis. I. Analysis with antibodies to adhesion molecules tenascin, N-CAM and integrin. Dev Biol 150:82–88
King IA, Tahiowo A, Williams RH (1980) Incorporation of L-(3H) fucose and D-(3H) glucosamine into cell surface associated glyco-conjugates in epidermis of cultured pig skin slices. Biochem J 190:65–77
Kitamura K (1987) The structure and distribution of proteochondroitin sulphate during the formation of chick embryo feather germs. Development 100:501–512
Kupchella CE, Matsouka LY, Bryan B, Wortsman J, Dietrich JG (1984) Histochemical evaluation of glycosaminoglycan deposition in the skin. J Histochem Cytochem 32:1121–1124
Lever-Fischer PL, Goetinck PF (1988) Identification and characterization of a proteoglycan in embryonic chicken skin that can interact with hyaluronic acid. Arch Biochem Biophys 263:45–48
Lockerbie RO (1987) The neuronal growth cone: a review of its locomotory, navigational and target recognition capabilities. Neuroscience 20:719–729
Mark MP, Baker JR, Kimata K, Ruch JV (1990 a) Regulated changes in chondroitin sulfation during embryogenesis: an immunohistochemical approach. Int J Dev Biol 34:191–204
Mark MP, Baker JR, Morrison K, Ruch JV (1990 b) Chondroitin sulfates in developing mouse tooth germs. An immunohistochemical study with monoclonal antibodies against chondroitin-4 and chondroitin-6-sulfates. Differentiation 43:37–50
Marsh RG, Gallin MJ (1992) Structural variants of the neural cell adhesion molecule (N-CAM) in developing feathers. Dev Biol 150:171–184
Martin P, Khan A, Lewis J (1989) Cutaneous nerves of the embryonic chick wing do not develop in regions denuded of ectoderm. Development 106:335–346
Mauger A, Demarchez M, Herbage D, Grimaud JA, Druguet M, Hartmann D, Sengel P (1982) Immunofluorescent localization of collagen types I and III and of fibronectin, during feather morphogenesis in the chick embryo. Dev Biol 94:93–105
McAdams BD, McLoon SC (1993) Influence of chondroitin sulfate on retinal axon growth in vitro. Soc Neurosci Abstr 19, p 1089
McLean IW, Nakane PK (1974) Periodate-lysine-paraformaldehyde fixative. A new fixative for immunoelectron microscopy. J Histochem Cytochem 22:1077–1083
Newgreen DF, Scheel M, Kastner V (1986) Morphogenesis of sclerotome and neural crest in avian embryos. In vivo and in vitro studies on the role of notochordal extracellular material. Cell Tissue Res 244:299–313
Oakley RA, Tosney KW (1991) Peanut agglutinin and chondroitin-6-sulfate are molecular markers for tissues that act as barriers to axon advance in the avian embryo. Dev Biol 147:187–207
Patterson PH (1988) On the importance of being inhibited, or saying no to growth cones. Neuron 1:263–267
Perkins ME, Ji TH, Hynes RO (1979) Crosslinking of fibronectin to sulfated proteoglycans at the cell surface. Cell 16:941–952
Perris R, Bronner-Fraser M (1989) Recent advances in defining the role of the extracellular matrix in neural crest development. Comments Dev Neurobiol 1:61–83
Perris R, Krotoski D, Lallier T, Domingo C, Sorrell JM, Bronner-Fraser M (1991) Spatial and temporal changes in the distribution of proteoglycans during avian neural crest development. Development 111:583–599
Reynolds ES (1963) The use of lead citrate at high pH as an electron dense stain in electron microscopy. J Cell Biol 17:208–212
Rich AM, Pearlstein E, Weissman OG, Hoffstein ST (1981) Cartilage proteoglycans inhibit fibronectin-mediated adhesion. Nature 293:224–226
Ring C, Halfter W, Lemmon V (1993) Two chondroitin sulfate proteoglycans expressed during chick embryo visual system development. Soc Neurosci Abstr 19:1086
Ripellino JA, Margolis RU, Margolis RK (1989) Immunoelectron microscopic localization of hyaluronic acid-binding region and link protein epitopes in brain. J Cell Biol 108:1899–1907
Saxod R (1978 a) Combination of cholinesterase staining of nerves and stereoscopic viewing for three-dimensional study of skin innervation on whole mounts. J Invest Dermatol 70:95–97
Saxod R (1978 b) Development of cutaneous sensory receptors in birds. In: Jacobson M (ed) Handbook of sensory physiology. Development of sensory systems, vol IX. Springer, Berlin, pp 337–417
Sengel P (1990) Pattern formation in skin development. Int J Dev Biol 34:33–50
Shinomura T, Jensen KL, Yamagata M, Kimata K, Solursh M (1990) The distribution of mesenchyme proteoglycan (PG-M) during wing bud outgrowth. Anat Embryol 181:227–233
Snow DM, Letourneau PC (1992) Neurite outgrowth on a step gradient of chondroitin sulfate proteoglycan. J Neurobiol 23:322–336
Snow DM, Lemmon V, Carrino DA, Caplan AI, Silver J (1990 a) Sullated proteoglycans in astroglial barriers inhibit neurite outgrowth in vitro. Exp Neurol 109:111–130
Snow DM, Steindler DA, Silver J (1990 b). Molecular and cellular characterization of the glial roof plate of the spinal cord and optic tectum: a possible role for a proteoglycan in the development of an axon barrier. Dev Biol 138:359–376
Snow DM, Watanabe M, Letourneau PC, Silver J (1991) A chondroitin sulfate proteoglycan may influence the direction of retinal ganglion cell outgrowth. Development 113:1473–1485
Sobue M, Takeuchi J, Fukatsu T, Nagasaka T, Nakashima N, Ogura T, Katoh T (1989) Immunohistochemical technique for detection of dermatan sulphate-proteoglycans in tissue sections. Stain Technol 64:43–47
Sorrell JM, Mahmoodian F, Schafer IA, Davis B, Caterson B (1989) Identification of monoclonal antibodies that recognize novel epitopes in native chondroitin/dermatan sulfate glycosaminoglycan chains: their use in mapping functionally distinct domains of human skin. J Histochem Cytochem 38:393–402
Tan SS, Crossin KL, Hoffman S, Edelman GM (1987) Asymmetric expression in somites of cytotactin and its proteoglycan ligand is correlated with neural crest cell distribution. Proc Natl Acad Sci USA 84:7977–7981
Tosney KW (1991) Cells and cell interactions that guide motor axons in the developing chick embryo. BioEssays 13:17–23
Tougard C, Picart R (1989) Immunocytochimie avant inclusion: tissues, cellules en culture. In: Calas A, Gounon P, Hernandez-Verdun D, Nicolas G, Reynès M, Tougard C (ed) Immunocytochimie, méthodes pratiques. Societé Française de Microscopie Electronique, Ivry, France, pp 5–17
Tsao CH, Eisenstein R (1981) Attachment of proteoglycans to collagen fibrils. Effect on human platelet aggregation. Lab Invest 45:450–455
Tucker RP (1991) The sequential expression of tenascin mRNA in epithelium and mesenchyme during feather morphogenesis. Roux's Arch Dev Biol 200:108–112
Tuckett F, Morriss-Kay G (1988) Alcian blue staining of glycosaminoglycan in embryonic material: effect of different fixatives. Histochem J 20:174–182
Verna JM (1985) In vitro analysis of interactions between sensory neurons and skin: evidence for selective innervation of dermis and epidermis. J Embryol Exp Morphol 86:53–71
Verna JM, Saxod R (1979 a) Co-cultures de neurones ganglionnaires spinaux et de mesenchyme dermique d'embryons d'oiseaux. Biol Cell 35:233–242
Verna JM, Saxod R (1979 b) Developpement de l'innervation cutanée chez le poulet: analyse ultrastructurale et quantitative. Arch Anat Micr Morph Exp 68:1–16
Verna JM, Usson Y, Saxod R (1986) Differential growth of sensory neurons in vitro in presence of dermis and epidermis. A quantitative time-lapse analysis. Cell Differ 18:183–188
Verna JM, Fichard A, Saxod R (1989) Influence of glycosaminoglycans on neurite morphology and outgrowth patterns in vitro. Int J Dev Neurosci 7:389–399
Willen MD, Sorrell JM, Lekan CC, Davis BR, Caplan AI (1991) Patterns of glycosaminoglycan/proteoglycan immunostaining in human skin during aging. J Invest Dermatol 96:968–974
Yamagata M, Yamada KM, Yoneda L, Suzuki S, Kimata K (1986) Chondroitin sulfate proteoglycan (PG-M-like proteoglycan) is involved in the binding of hyaluronic acid to cellular fibronectin. J Biol Chem 261:13526–13535
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hemming, F.J., Pays, L., Soubeyran, A. et al. Development of sensory innervation in chick skin: comparison of nerve fibre and chondroitin sulphate distributions in vivo and in vitro. Cell Tissue Res 277, 519–529 (1994). https://doi.org/10.1007/BF00300225
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00300225