Abstract
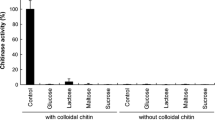
The anaerobic rumen fungus, Piromyces communis OTS1, was isolated from a fistulated goat. Its chitinolytic activity in the supernatant of media containing different types of chitin was studied. The fungus grew well in our basal medium, with and without colloidal chitin and chitin powder. N-Acetyl-β-glucosaminidase activity was not detected in any of the culture media. Chitinase activity, however, was detected in the basal medium with and without colloidal chitin and chitin powder. The extracellular chitinase concentrated from the fungal culture's supernatant by ammonium sulfate (80% saturation) showed highest activity at 40°C and at pH 5.5. In the other cell fractions of P. communis OTS1, N-acetyl-β-glucosaminidase was not detected, but chitinase activity was detected by 4-methylumbelliferyl reagents. Thus, it was found that the rumen fungus P. communis OTS1 has chitinase activity. Chitinases from the extracellular, cytosolic, and the microsomal fractiòns were mainly of the endo type of chitinase activity.
Similar content being viewed by others

Literature Cited
Balasubramanian R, Manocha MS (1992) Cytosolic and membrane-bound chitinases of two mucoraceous fungi: a comparative study. Can J Microbiol 38:331–338
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Cabib E (1986) The synthesis and degradation of chitin. Adv Enzymol 59:59–101
Gooday GW (1990) Chitinases. In: Leatham G, Himmel M (eds) Enzymes in biomass conversion. Washington, DC: American Chemical Society, pp 478–485
Jeuniaux C (1966) Chitinases In: Neufeld EF, Ginsburg V (eds) Complex carbohydrates. New York: Academic Press Inc., pp 645–650
Lowe SE, Theodorou MK, Trinci APJ (1987a) Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl Environ Microbiol 53:1216–1223
Lowe SE, Theodorou MK, Trinci APJ (1987b) Growth and fermentation of an anaerobic rumen fungus on various carbon sources and effect of temperature on development. Appl Environ Microbiol 53:1210–1215
Morgavi DP, Onodera R, Nagasawa T (1993) In vitro metabolism of chitin and protein from ruminal fungi by ruminal protozoa. Anim Sci Technol (Jpn) 64:584–592
Morgavi DP, Sakurada M, Tomita Y, Onodera R (1994) Presence in rumen bacterial and protozoal populations of enzymes capable of degrading fungal cell walls. Microbiology 140:631–636
Onodera R, Henderson C (1980) Growth factors of bacterial origin for the culture of the rumen oligotrich protozoom, Entodinium caudatum. J Appl Bacteriol 48:125–134
Orpin CG (1977) The occurrence of chitin in the cell walls of the rumen organisms Neocallimastix frontalis, Piromonas communis and Sphaeromonas communis. J Gen Microbiol 99:215–218
Patton RS, Chandler PT (1975) In vivo digestibility evaluation of chitinous materials. J Dairy Sci 58:397–401
Robbins PW, Albright C, Benfield B (1988) Cloning and expression of a Streptomyces plicatus chitinase (chitinase-63) in Escherichia coli. J Biol Chem 263:443–447
Sakurada M, Tsuzuki Y, Morgavi DP, Tomita Y, Onodera R (1995) Simple method for cryopreservation of an anaerobic rumen fungus using ethylene glycol and rumen fluid. FEMS Microbiol Lett 127:171–174
Shimahara K, Takiguchi Y (1988) Preparation of crustacean chitin. In: Wood WA, Kellogg ST (eds) Biomass part B: lignin, pectin, and chitin. San Diego, Calif.: Academic Press, Inc., pp 417–423
Steat R, Mah RA (1984) Quantitative method for colorimetric determination of formate in fermentation media. Appl Environ Microbiol 47:884–885
Stoscheck CM (1990) Quantitation of protein. Methods Enzymol 182:50–68
Tsujibo H, Yoshida Y, Miyamoto K, Imada C, Okami Y, Inamori Y (1992) Purification, properties, and partial amino acid sequence of chitinase from a marine Alteromonas sp. strain O-7. Can J Microbiol 38:891–897
Ueda M, Arai M (1992) Purification and some properties of chitinases from Aeromonas sp. No. 10S-24. Biosci Biotechnol Biochem 56:460–464
Wang Q, Zhou Z-Y, Sakuda S, Yamada Y (1993) Purification of allosamidin-sensitive and-insensitive chitinases produced by allosamidin-producing Streptomyces. Biosci Biotechnol Biochem 57:467–470
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sakurada, M., Morgavi, D.P., Tomita, Y. et al. Chitinolytic activity of the anaerobic rumen fungus Piromyces communis OTS1. Current Microbiology 31, 206–209 (1995). https://doi.org/10.1007/BF00298374
Issue Date:
DOI: https://doi.org/10.1007/BF00298374