Summary
Previous publications suggest that prolonged inhalation of frusemide (F) does not cause a fall in the nasal transepithelial potential difference (PD) whereas locally deposited F does. In an attempt to reconcile these observations, we have measured the effect of inhalation through the nose and local deposition of F, amiloride (A), bumetanide (B) and salbutamol (S) on nasal PD in 7 healthy male volunteers in a randomised, double blind study. Solutions of drugs ranging from 10−6 M to 10−3 M (3.10−8 M to 3.10−5 M for B) in phosphate buffered saline 0.5 ml (PBS) were sequentially deposited in both nostrils, and nasal PD was measured 5 min after each dose. In 10 further volunteers, 10−2 M solutions of A, F and S (3.10−4 M for B) 5 ml were nebulised through the nose for 15 min, when nasal PD was measured.
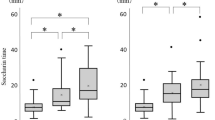
Resting PD was similar in the left and right nostrils averaging −17.1 mV (lumen negative). Placebo, inhaled of deposited B and S, and inhaled F did not change nasal PD. Topically deposited F significantly lowered PDmax in a dose-dependent manner [10−4 M, −12% from baseline; 10−3 M, −24%] as did the more potent A [10−5 M, −19%; 10−4 M, −31%; 10−3 M, −47%]. Nebulised A (10−2 M) had the same effect on nasal PD as deposited A (10−4 M). The effects of locally deposited F and A (10−3 M) on nasal PD were additive.
Our results suggest first that, aerosol administration is less effective than local application in assessing the effect of a drug on nasal PD. And second, F cannot act primarily at the cotransporter level to reduce nasal PD, as B and F share the same inhibitory effect of the basolateral Na/K/Cl cotransporter and B does not reduce nasal PD.
Similar content being viewed by others
References
Acker GM, Molimard M, Regnard J, Naline E, Freche C, Lochkart A (1993) Effect of frusemide on prostaglandin synthesis by human nasal and bronchial cells in culture. Am J Resp Cell Mol Biol (in press)
Alton EWFW, Currie D, Logan-Sinclair R, Warner JO, Hodson ME, Geddes DM (1990) Nasal potential difference: a clinical diagnostic test for cystic fibrosis. Eur Resp J 3: 922–926
Anderson MP, Welsh MJ (1990) Isoproterenol, cAMP, and bradykinin stimulate diacylglycerol production in airway epithelium. Am J Physiol 258: L294-L300
Anderson SD, He W, Temple DM (1991) Inhibition by frusemide of inflammatory mediators from lung fragments (letter). N Engl J Med 324: 131
Bianco SA, Vaghi A, Robuschi M, Pasargiklian M (1988) Prevention of exercise-induced bronchoconstriction by inhaled frusemide. Lancet II: 252–255
Bianco S, Pieroni MG, Refini M, Sturman A, Robuschi M (1989) Protective effect of inhaled frusemide on allergen-induced early and late asthmatic reactions. N Engl J Med 321: 1069–1073
Downey GP, Gumbay RS, Doherty DE, Labreque JF, Henson JE, Henson PM, Worthen GS (1988) Enhancement of pulmonary inflammation by PGE2: evidence for a vasodilator effect. J Appl Physiol 64: 728–741
Duggan CJ, Dixon CMS, Ind PW (1990) The effect of frusemide and bumetamide in exercise-induced asthma (abstract). Am Rev Resp Dis 141: A474
Evans MG, Marty A, Tan YP, Trautman A (1986) Blockade of Ca-activated CI conductance by frusemide in rat lacrymal glands. Pflug Arch 406: 65–68
Fujimura M, Sakamoto S, Kamio Y (1990) Effect of inhaled frusemide on bronchial responsiveness to methacholine. N Engl J Med 322: 935–936
Greger R (1988) Chloride transport in thick ascending limb, distal convolution and collecting duct. Ann Rev Physiol 50: 111–122
Horrobin DF, Manku MS, Mtabaji JP (1976) Vascular actions of frusemide and bumetanide on the rat superior mesenteric vascular bed: interactions with prostaglandins. Clin Sci Mol Med 51 [Suppl 3]: S257-S258
Johnston GD, Nicholls DP, Kondowe GB, Finch MB (1986) Comparison of the acute vascular effects of frusemide and bumetanide. Br J Clin Pharmacol 21: 359–364
Katayama S, Attallah AA, Stahl RAK, Bloch DL, Lee JB (1984) Mechanism of frusemide-induced natriuresis by direct stimulation of renal prostaglandin E2. Am J Physiol 247 F555-F561
Knowles MR, Carlson JL, Collier AM, Gatzy JT, Boucher RC (1981) Measurements of nasal transepithelial electric potential differences in normal human subjects in vivo. Am Rev Resp Dis 124: 484–490
Knowles MR, Buntin WH, Bromberg PA, Gatzy JT, Boucher RC (1982) Measurements of transepithelial electric potential difference in the trachea and bronchi of human subjects in vivo. Am Rev Resp Dis 126: 108–112
Knowles MR, Murray G, Shallal J, Askin F, Ranga V, Gatzy JT, Boucher RC (1984) Bioelectric properties and ion flow across excised human bronchi. J Appl Physiol 56: 868–877
Knowles MR, Church NL, Waltner WE, Yankaskas JR, Gilligan P, King M, Edwards LJ, Helms RW, Boucher RC (1990) A pilot study of aerosolized amiloride for the treatment of lung disease in cystic fibrosis. N Engl J Med 322: 1189–1194
Lundergan FC, Fitzpatrick TM, Rose JC, Ramwell PW, Knot PA (1988) Effect of cyclooxygenase inhibition on the pulmonary vasodilator response to frusemide. J Pharmacol Exp Ther 246: 102–106
McFadden ER Jr (1992) Microvasculature and airway responses. Am Rev Resp Dis 145: S42-S43
McCann JD, Welsh MJ (1990) Regulation of CI− and K+ channels in airway epithelium. Ann Rev Physiol 52: 115–135
Morrow PE, Yu CP (1985) Models of aerosol behavior in airways. In: Morén F, Newhouse MT, Dolovich MB (eds) Aerosols in medicine, principles, diagnosis and therapy. Elsevier, Amsterdam
Nichol GM, Alton EWFW, Nix A, Geddes DM, Chung KF, Barnes PJ (1990) Effect of inhaled frusemide on metabisulfite- and methacholine-induced bronchoconstriction and nasal potential difference in asthmatic subjects. Am Rev Resp Dis 142: 576–580
O'Connor BJO, Ridge S, Chung KF, Fuller RW, Barnes PJ (1991) Effect of inhaled frusemide and bumetanide on adenosine 5′-monophosphate- and sodium metabisulfite-induced bronchoconstriction in asthmatic subjects. Am Rev Resp Dis 143: 1329–1333
Polosa R, Lau LCK, Holgate ST (1990) Inhibition of adenosine 5′-monophosphate-and methacholine-induced bronchocon-striction in asthma by inhaled frusemide. Eur Resp J 3: 665–672
Robuschi MG, Gambard G, Spägnotto S, Vaghi A, Bianco S (1989) Inhaled frusemide is highly effective in preventing ultrasonically nebulized water bronchoconstriction. Pulm Pharmacol 1: 187–191
Salah B, Dinh-Xuan AT, Fouilladieu JL, Lockhart A, Regnard J (1988) Nasal mucociliary transport in healthy subjects is slower when breathing dry air. Eur Resp J 1: 852–855
Ward A, Heel RC (1984) Bumetanide: a review of its pharmacodynamic and pharmacokinetic properties and therapeutic use. Drugs 28: 426–464
Widdicombe JH, Nathanson IT, Highland E (1983) Effects of loop diuretics on ion transport by dog epithelium. Am J Physiol 245: C388-C396
Widdicombe JH, Ueki IF, Emery D, Margolskee D, Yerget J, Nadel JA (1989) Release of cyclooxygenase products from primary cultures of tracheal epithelia of dog and human. Am J Physiol 257: L361-L365
Willumsen NJ, Davis CW, Boucher RC (1989) Intracellular CI-activity and cellular. CI-pathways in cultured human airway epithelium. Am J Physiol 256: C1033-C1044
Wood AM, Higgenbottam TW (1988) Nasal potential difference: effect of aerosol challenge of varying tonicity and ionic content (abstract). Thorax 43: 234p
Wood AM, Dinh-Xuan AT, Cremona G, Lockhart A, Higgenbottam TW (1991) The α1-adrenergic agonist methoxamine and the “loop” diuretic frusemide reduce nasal potential difference. Eur Resp J 4: 802–806
Wood AM, Lowry RH, Higgenbottam TW (1990) Differential effects of inhaled frusemide and amiloride on airway responsiveness to water and exercise in asthma (abstract). Am Rev Resp Dis 141: A478
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mialon, P., Charfi, R., Regnard, J. et al. Locally deposited but not inhaled frusemide reduces nasal potential difference in healthy subjects. Eur J Clin Pharmacol 45, 347–351 (1993). https://doi.org/10.1007/BF00265953
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00265953