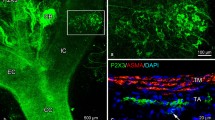
Abstract
In order to clarify the developmental pattern in the sympathetic nerve fibers innervating the cerebral arterial system during the postnatal period in rats, we labeled the postganglionic nerve fibers originating in the superior cervical ganglion (SCG) and directly observed their extension and plexus formation by means of anterograde labeling with wheat germ agglutinin-horseradish peroxidase (WGA-HRP). The WGA-HRP solution was injected into the right SCG 1–7 days after birth. The rats were killed 48 h after trace injection, and the cerebral arteries were reacted with tetramethylbenzidine, then observed as a whole mount preparation. The labeled nerve fibers appeared as a few relatively straight bundles with branching fibers running longitudinally to the long axis of the artery in the ipsilateral right side of the circle of Willis and proximal portion of their main branching arteries at 3 days after birth. The nerve fibers started to form a circular pattern of nerve plexus only on the wall of the circle of Willis as early as 1 week after birth. At the beginning of postnatal week 2, labeled nerve fibers extended the collateral projections into the collateral side of the circle of Willis, and these expanding projections could not be observed at postnatal week 3. We observed a route of the sympathetic nerve fibers advancing into the cerebral arterial system which has not been described in previous studies; bundle of labeled nerve fibers entered into the wall of the middle portion of the basilar artery in half of the animals, in any postnatal period. We were able to confirm, by using an anterograde labeling technique with WGA-HRP, how the sympathetic nerve fibers advance into the cerebral arterial system, when they start to form nerve plexus during the postnatal period in rats, and clarified that the sympathetic nerve fibers showed overabundant collateral projection in the cerebral arterial system during the early postnatal period.
Similar content being viewed by others
References
Arbab MAR, Wiklund L, Delgado T, Svendgaard NA (1988) Stellate ganglion innervation of the vertebro-basilar arterial system demonstrated in the rat with anterograde and retrograde WGA-HRP tracing. Brain Res 445:175–180
Allen JM, Schon F, Todd N, Yeates JC, Crockard HA, Bloom SR (1984) Presence of neuropeptide Y (NPY) in human circle of Willis and its possible role in cerebral vasospasm. Lancet 2:550–552
Bennett MR, Lavidis NA (1984) Development of the topographical projection of motor neurons to a rat muscle accompanies loss of polyneuronal innervation. J Neurosci 4:2204–2212
Bevan RD (1984) Trophic effects of peripheral a vascular structure. Hypertension [Suppl III] pp 19–26
Brown MC, Jansen JKS, Van Essen D (1976) Polyneuronal innervation of skeletal muscle in new-born rats and its elimination during maturation. J Physiol 261:387–422
Cowan WM, Fawcett JW, O'Leary DDM, Stanfield BB (1984) Regressive events in neurogenesis. Science 225:1258–1265
Edvinsson L, Emson P, McCulloch J, Tatemoto K, Uddman R (1984) Neuropeptide Y: immunocytochemical localization to and effect upon feline pial arteries and veins in vitro and in situ. Acta Physiol Scand 122:155–163
Falck B, Mchedlishvili GI, Owman C (1965) Histochemical demonstration of adrenergic nerves in cortex-pia of rabbit. Acta Pharmacol Toxicol 23:133–142
Handa Y, Caner H, Hayashi M, Tamamaki N, Nojyo Y (1990) The distribution pattern of the sympathetic nerve fibers to the cerebral arterial system in rats as revealed by anterograde labeling with WGA-HRP. Exp Brain Res 82:493–498
Handa Y, Nojyo Y, Hayashi M (1991) Patterns of reinnervation of denervated cerebral arteries by sympathetic nerve fibers after unilateral ganglionectomy in rats. Exp Brain Res 86:82–89
Handa Y, Nojyo Y, Ishiguro H, Nagatsu I (1992) Plasticity of the sympathetic nervous system innervating the cerebral arteries in rats. Exp Neurol 118:324–331
Heisted DD, Marcus ML, Abbound FM (1978) Experimental attempts to unmask effects of neural stimuli on cerebral blood flow. In: Purves MJ (eds) Cerebral vascular smooth muscle and its control. Elsevier, Amsterdam, pp 97–111
Hill CE, Vidovic M (1989) The role of competition in the refinement of the projections of sympathetic neurons to the rat eye during development. Int J Dev Neurosci 7:539–551
Hubel DH, Wiesel TN, LeVay S (1977) Plasticity of ocular dominance columns in monkey striate cortex. Philos Trans R Soc Lond [Biol] 278:377–409
Jackson H, Parks TN (1982) Functional synapse elimination in the developing avian cochlear nucleus with simultaneous reduction in cochlear nerve axon branching. J Neurosci 2:1736–1743
Jackson PC (1983) Reduced activity during development delays the normal rearrangement of synapses in the rabbit ciliary ganglion. J Physiol 345:319–327
Johnson DA, Purves D (1981) Post-natal reduction of neural unit size in the rabbit ciliary ganglion. J Physiol 318:143–159
Kajikawa H (1969) Mode of the sympathetic innervation of the cerebral vessels demonstrated by the fluorescent histochemical technique in rats and cats. Arch Jpn Chir 38:227–235
Lichtman JW (1977) The reorganization of synaptic connexions in the rat submandibular ganglion during post-natal development. J Physiol 273:155–177
Lichtman JW, Purves D (1980) The elimination of redundant preganglionic innervation to hamster sympathetic ganglion cells in early post-natal life. J Physiol 301:213–228
Mesulam MM (1978) Tetramethylbenzidine for horseradish peroxidase neurohistochemistry: a non-carcinogenic blue reaction-product with superior sensitivity for visualizing neural afferents and efferents. J Histochem Cytochem 26:106–117
Nielsen KC, Owman C (1967) Adrenergic innervation of pial arteries related to the circle of Willis in the cat. Brain Res 6:773–776
Nojyo Y, Tamamaki N, Matsuura T, Sano Y (1988) Histochemical and electron microscopical demonstration of the sympathetic nerve fibers joining to the fourth and the sixth cranial nerves in rats. Histochemistry 88:557–561
Purves D, Lichtman JW (1980) Elimination of synapses in the developing nervous system. Science 210:153–157
Redfern PA (1970) Neuromuscular transmission in new-born rats. J Physiol 209:701–709
Tamamaki N, Nojyo Y (1987) Intracranial trajectories of sympathetic nerve fibers originating in the superior cervical ganglion in the rat: WGH-HRP anterograde labeling study. Brain Res 437:387–392
Thompson W (1983) Synapse elimination in neonatal rat muscle in sensitive to pattern of muscle use. Nature 302:614–616
Tolbert DL (1987) Intrinsically directed pruning as a mechanism regulating the elimination of transient collateral pathway. Brain Res Dev Brain Res 33:11–21
Tsai SH, Tew JM, Shipley MT (1989) Cerebral arterial innervation II. Development of calcitonin-gene-related peptide and norepinephrine in the rat. J Comp Neurol 279:1–12
Vidovic M, Hill C (1988) Withdrawal of collaterals of sympathetic axons to the rat eye during postnatal development: the role of function. J Auton Nerv Syst 22:57–65
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Handa, Y., Nojyo, Y., Tamamaki, N. et al. Development of the sympathetic innervation to the cerebral arterial system in neonatal rats as revealed by anterograde labeling with wheatgerm agglutinin-horseradish peroxidase. Exp Brain Res 94, 216–224 (1993). https://doi.org/10.1007/BF00230289
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230289