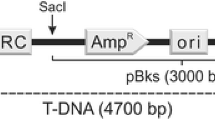
Abstract
DNA sequences of the basidiomycete Agrocybe aegerita were cloned in E. coli based on their ability to drive the expression of the bacterial promoterless tetracycline (Tc)-resistance gene. A 0.48% frequency of the cloned sequences promoted antibiotic-resistance. The sequence conferring the highest Tc resistance (40 μg/ml) was selected to drive the expression in E. coli of two other promoterless genes encoding chloramphenicol and neomycin resistance. One of the derivative vectors, pN13-A2, carrying a chimeric neomycin-resistance gene, was used to transform an A. aegerita neomycin-sensitive strain by protoplast electroporation. Transformation frequencies ranged from 1 to 2.8 transformants per μg of DNA per 103 viable cells, in a relatively high background of spontaneous-resistant colonies (2% of the surviving protoplasts). Molecular analyses showed that transformation had occurred by the integration of pN13-A2 sequences, either ectopically or at the resident locus carrying the A. aegerita promoter-like sequence, with probable molecular rearrangements. The nucleotide sequence of the promoter-like fragment revealed the presence of a CT motif that is known to be involved in a promoter function in some highly expressed genes of filamentous fungi.
Similar content being viewed by others
References
Barret V, Dixon RK, Lemke PA (1990) Genetic transformation of a mycorrhizal fungus. Appl Microbiol Biotechnol 33:313–316
Binninger DM, Skrzynia C, Pukkila PJ, Casselton LA (1987) DNAmediated transformation of the basidiomycete Coprinus cinereus. EMBO J 6:835–840
Bolivar F, Rodriguez RL, Greene PJ, Betlach MC, Heynecker HL, Boyer HW, Crosa JH, Falkow S (1977). Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95–113
Brosius J (1984). Plasmid vector for the selection of promoters. Gene 27:151–160
Fincham JRS (1989) Transformation in fungi. Microbiol Rev 53:148–170
Gorman C, Padmanabhan R, Howard BH (1983) High-efficiency DNA-mediated transformation of primate cells. Science 221:551–553
Gurr SJ, Unkles SE, Kinghorn JR (1987) The structure and organization of nuclear genes of filamentous fungi. In: Kinghorn JR (ed) Gene structure in eukaryotic microbes. IRL Press, Oxford Washington, pp 93–139
Hanahan D (1985) Techniques for transformation of Escherichia coli. In: Glover DM (ed) DNA cloning: a practical approach, vol 1. IRL Press, Oxford Washington, pp 1–47
Herman LMF, Van Montagu MC, Depicker AG (1986) Isolation of tobacco DNA segments with plant promoter activity. Mol Cell Biol 6:4486–4492
Hirth KP, Edwards CA, Firtel RA (1982) A DNA-mediated transfermation system for Dictyostelium discoideum. Proc Natl Acad Sci USA 79:7356–7360
Jimenez A, Davies J (1980) Expression of a transposable antibiotic resistance element in Saccharomyces. Nature 287:869–871
Labarère J, Noël T, Imbernon M (1989) Selection and genetic analysis of antibiotic-resistant mutant strains in Agrocybe aegerita. Mush Sci XII 1:161–174
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York
Marmeisse R, Gay G, Debaud JC, Casselton LA (1992) Genetic transformation of the symbiotic basidiomycete fungus Hebeloma cylindrosporum. Curr Genet 22:41–45
Mooibroek H, Kuipers AGJ, Siestsma JH, Punt PJ, Wessels JGH (1990) Introduction of hygromycin B resistance into Schizophyllum commune: preferential methylation of donor DNA. Mol Gen Genet 222:41–48
Noël T, Labarère J (1989) Isolation of DNA from Agrocybe aegerita for the construction of a genomic library in Escherichia coli. Mush Sci XII 1:187–201
Noël T, Labarère J (1994) Homologous transformation of the edible basidiomycete Agrocybe aegerita with the URA1 genexharacterization of integrative events and of rearranged free plasmids in transformants. Curr Genet 25:432–437
Noël T, Ho Huynh TD, Labarère J (1991) Genetic variability of the wild incompatibility alleles of the tetrapolar basidiomycete Agrocybe aegerita. Theor Appl Genet 81:745–751
Peng M, Singh NA, Lemke PA (1992) Recovery of recombinant plasmids from Pleurotus ostreatus transformants. Curr Genet 22:53–59
Punt PJ, Oliver RP, Dingemanse MA, Pouwels PH, van den Hondel CAMJJ (1987) Transformation of Aspergillus based on the hygromycin-B resistance marker from Escherichia coli. Gene 56:117–124
Randall T, Reddy CA, Boominathan K (1991) A novel extrachromosomally maintained transformation vector for the lignin-degrading basidiomycete Phanerochaete chrysosporium. J Bacteriol 173:776–782
Raper JR, RM Hoffman (1974) Schizophyllum commune. In: King RC (ed) Handbook of genetics. Plenum Press, New York, pp 597–626
Razanamparany V, Bégueret J (1988) Non-homologous integration of transforming vectors in the fungus Podospora anserina: sequences of junctions at the integration sites. Gene 74:399–409
Sanger F, Nieklen S, Coulson AR (1977) DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA 74: 5463–5467
Simoneau P, Labarère J (1990) Construction of chimeric antibioticresistance determinants and their use in the development of cloning vectors for spiroplasmas. Zentralbl Mikrobiol 20:66–74
Southern PJ, Berg P (1982) Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet 1:327–341
Suarez T, Eslava AP (1988) Transformation of Phycomyces with a bacterial gene of kanamycin resistance. Mol Gen Genet 212:120–123
Teeri TH, Herrera-Estrella L, Depicker A, Van Montagu M, Palva ET (1986) Identification of plant promoters in situ by T-DNAmediated transcriptional fusions to the npt-II gene. EMBO J 5:1755–1760
Wang J, Holden DW, Leong SA (1988) Gene transfer system for the phytopathogenic fungus Ustilago maydis. Proc Natl Acad Sci USA 85:865–869
Webster TD, Dickson RC (1983) Direct selection of Saccharomyces cerevisiae resistant to the antibiotic G418 following transformation with a DNA vector carrying the kanamycin resistance gene of Tn903. Gene 26:243–252
West RW, Neve RL, Rodriguez RL (1979) Construction and characterization of E. coli promoter-probe plasmid vectors. I. Cloning of promoter-containing DNA fragments. Gene 7:271–288
Author information
Authors and Affiliations
Additional information
Communicated by L. Alföldi
Rights and permissions
About this article
Cite this article
Noël, T., Simoneau, P. & Labarère, J. Heterologous transformation of Agrocybe aegerita with a bacterial neomycin-resistance gene fused to a fungal promoter-like DNA sequence. Theoret. Appl. Genetics 90, 1019–1027 (1995). https://doi.org/10.1007/BF00222916
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00222916