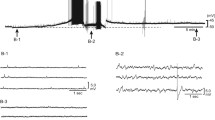
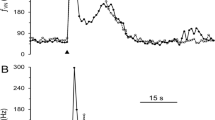
Abstract
The ATP-induced increase in tritium outflow from cultured chick sympathetic neurons prelabelled with [3H]-noradrenaline was investigated.
Seven days-old dissociated cell cultures of embryonic paravertebral ganglia, loaded with [3H]-noradrenaline (0.05 μM), were superfused in the presence of (+)-oxaprotiline and exposed to ATP, ATP-analogues, or 1,1-dimethyl-4-piperazinium (DMPP) for 2 min. ATP (3 μLM-3 mM), 2-methylthio-ATP (3–100 μM), as well as DMPP (10 and 100 μM) induced a significant overflow of tritium. The EC50-value of ATP was 20 μM. Both the ATP-induced and the DMPP-induced tritium overflow was Ca2+-dependent and sensitive to tetrodotoxin (0.3 μM) and ω-conotoxin (0.1 μM); in addition, it was inhibited by the α2-adrenoceptor agonist 5-bromo-6-(2-imidazoline-2-ylamino)-quinoxaline (UK-14,304; 1 μM). The effects of ATP and DMPP were not additive. The ATP-induced as well as the DMPP-induced overflow of tritium was diminished by the P2-purinoceptor antagonists suramin (300 μM) and reactive blue 2 (3 μM); in all 4 cases, the inhibition amouted to approximately 40%. The tritium overflow induced by ATP or DMPP was almost abolished by the nicotinic receptor antagonist mecamylamine (10 μM) and markedly inhibited by hexamethonium (100 μM). Neither ATP nor electrical stimulation caused an overflow of tritium from cultures loaded with [3H]-choline.
The results suggest that ATP at μmolar concentrations induces noradrenaline release from cultured chick sympathetic neurons via an action on a subclass of the nicotinic cholinoceptor.
Similar content being viewed by others
References
Akasu T, Koketsu K (1985) Effect of adenosine triphosphate on the sensitivity of the nicotinic acetylcholine-receptor in the bullfrog sympathetic ganglion cell. Br J Pharmacol 84:525–531
Allgaier C, Choi KB, Hertting G (1993) Muscarine receptors regulating electrically evoked release of acetylcholine in hippocampus are linked to pertussis toxin-sensitive G-proteins but not to adenylate cyclase. J Neurochem 61:1043–1049
Allgaier C, Pullmann F, Schobert A, von Kügelgen I, Heating G (1994a) P2 Purinoceptors modulating noradrenaline release from sympathetic neurons in culture. Eur J Pharmacol 252:R7-R8
Allgaier C, Schobert A, Belledin M, Jackisch R, Hertting G (1994b) Modulation of electrically evoked [3H]-noradrenaline release from cultured chick sympathetic neurons. Naunyn-Schmiedeberg's Arch Pharmacol 350:258–266
Allgaier C, Wellmann H, Schobert A, von Kügelgen I (1995) Cultured chick sympathetic neurons: modulation of electrically evoked noradrenaline release by P2-purinoceptors. Naunyn-Schmiedeberg's Arch Pharmacol, 352:17–24
Ascher P, Large WA, Rang HP (1979) Studies on the mechanism of action of acetylcholine antagonists on rat parasympathetic ganglion cells. J Physiol 295:139–170
Boehm S (1994) Noradrenaline release from rat sympathetic neurons evoked by P2-purinoceptor activation. Naunyn-Schmiedeberg's Arch Pharmacol 350:454–458
Böhm S, Huck S, Drobny H, Singer EA (1991) Electrically evoked noradrenaline release from cultured chick sympathetic neurons: modulation via presynaptic α2-adrenoceptors and lack of autoinhibition. Naunyn-Schmiedeberg's Arch Pharmacol 344:130–132
Bruns RE, Lu GH, Pugsley TA (1986) Characterization of the A2 adenosine receptor labeled by [3H]NECA in rat stritial membranes. Mol Pharmacol 29:331–346
Burnstock G, Kennedy C (1985) Is there a basis for distinguishing two types of P2 purinoceptor? Gen Pharmacol 16:433–440
Burnstock G, Warland J (1987) P2-Purinoceptors of two subtypes in the rabbit mesenteric artery: reactive blue 2 selectively inhibits responses mediated via the P2y - but not the P2x -purinoceptor. Br J Pharmacol 90: 383–391
Cambridge D (1981) UK-14,304, a potent and selective α2-agonist for the characterization of α-adrenoceptor subtypes. Eur J Pharmacol 72:413–415
Clones R, Jones S, Brown DA (1993) Zn2+ potentiates ATP-activated currents in rat sympathetic neurons. Pfügers Arch 424:152–158
Connolly GP, Harrison PJ, Stone TW (1993) Action of purine and pyrimidine nucleotides on the rat superior cervical ganglion. Br J Pharmacol 110:1297–1304
Edwards FA, Gibb AJ (1993) ATP — a fast neurotransmitter. FEBS Lett 325:86–89
Edwards FA, Gibb AJ, Coloquhoun D (1992) ATP receptor-mediated synaptic currents in the central nervous system. Nature 359:144–147
Evans RJ, Derkarch V, Suprenant A (1992) ATP mediates fast synaptic transmission in mammalian neurons. Nature 357: 503–505
Fieber LA, Adams DJ (1991) Adenosine triphosphate-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol 434:239–256
Greene LA, Rein G (1978) Release of norepinephrine from neurons in dissociated cell cultures of chick sympathetic ganglia via stimulation of nicotinic and muscarinic acetylcholine receptors. J Neurochem 30:579–586
Igusa Y (1988) Adenosine 5′-triphosphate activates acetylcholine receptor channels in cultured Xenopus myotomal muscle cells. J Physiol 405:169–185
Illes P, Nörenberg W (1993) Neuronal ATP receptors and their mechanism of action. Trends Pharmacol Sci 14:50–54
Inoue K, Nakazawa K, Fujimori K, Tanaka A (1989) Extracellular adenosine 5′-triphosphate-evoked norepinephrine secretion not relating to voltage-gated Ca channels in pheochromocytoma PC12 cells. Neurosci Lett 106:294–299
Inoue K; Nakazawa K, Fujimori K, Watano T, Tanaka A (1992) Extracellular adenosine 5′-triphosphate-evoked glutamate release in cultured hippocampal neurons. Neurosci Lett 134:215–218
Jahr CE, Jessell TM (1983) ATP excites a subpopulation of rat dorsal horn neurones. Nature 304:730–733
Katayama Y, Morita K (1989) Adenosine 5′-triphosphate modulates membrane potassium conductance in guinea-pig myenteric neurones. J Physiol 408:373–390
Kennedy C (1990) P1- and P2-purinoceptor subtypes — an update. Arch Int Pharmacodyn 303:30–50
Kim KT, Westhead EW (1989) Cellular responses to Ca2+ from extracellular and intracellular sources are different as shown by simultaneous measurements of cytosolic Ca2+ and secretion from bovine chromaffin cells. Proc Natl Acad Sci USA 86:9881–9885
Löffelholz K (1979) Release induced by nicotinic agonists. In: Paton DM (ed) The release of catecholamines from adrenergic neurons. Pergamon Press, Oxford, pp 275–301
Moody C, Burnstock G (1982) Evidence of the presence of P1-purinoceptors on cholinergic nerve terminals in the guinea pig ileum. Eur J Pharmacol 77:1–9
Nakazawa K (1994) ATP-activated current and its interaction with acetylcholine-activated current in rat sympathetic neurons. J Neurosci 14:740–750
Nakazawa K, Inoue K (1993) ATP- and acetylcholine-activated channels co-existing in cell-free membrane patches from rat sympathetic neuron. Neurosci Lett 163:97–100
Phillis JW, Kostopoulos GK, Limacher JJ (1975) A potent depressant action of adenine derivatives on cerebral cortical neurones. Eur J Pharmacol 30:125–129
Reekie FM, Burnstock G (1994) Some effects of purines on neurones of guinea-pig superior cervical ganglia. Gen Pharmacol 25: 143–148
Salt TE, Hill RG (1983) Excitation of single sensory neurones in the rat caudal trigeminal nucleus by iontophoretically applied adenosine 5′-triphosphate. Neurosci Lett 35:53–57
Shen K-Z, North RA (1993) Excitation of rat locus coeruleus neurons by adenosine 5′-triphosphate: ionic mechanism and receptor characterization. J Neurosci 13:894–899
Silinsky EM, Gerzanich V, Vanner SM (1992) ATP mediates excitatory synaptic transmission in mammalian neurones. Br J Pharmacol 106:762–763
Starke K (1977) Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol 77:1–124.
Tschöpl M, Harms L, Nörenberg W, Illes P (1992) Excitatory effects of adenosine 5′-triphosphate on rat locus coeruleus neurones. Eur J Pharmacol 213:71–77
von Kügelgen I, Schöffel E, Starke K (1989) Inhibition by nucleotides acting at presynaptic P2-receptors of sympathetic neuroeffector transmission in the mouse isolated vas deferens. Naunyn-Schmiedeberg's Arch Pharmacol 340:522–532
Varanda WA, Aracava Y, Sherby SM, van Meter WG, Eldefrawi ME, Albuquerque EX (1985) The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol Pharmacol 28:128–137
Wakade AR, Wakade TD (1988) Comparison of transmitter release properties of embryonic sympathetic neurons growing in vivo and in vitro. Neuroscience 27:1007–1019
Waldmeier PC, Baumann PA, Hauser K, Maitre L, Storm A (1982) Oxaprotiline, a noradrenaline uptake inhibitor with an active and an inactive enantiomer. Biochem Pharmacol 31:2169–2176.
Zimmermann H (1994) Signalling via ATP in the nervous system. Trends Neurosci 17:420–426
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Allgaier, C., Wellmann, H., Schobert, A. et al. Cultured chick sympathetic neurons: ATP-induced noradrenaline release and its blockade by nicotinic receptor antagonists. Naunyn-Schmiedeberg's Arch Pharmacol 352, 25–30 (1995). https://doi.org/10.1007/BF00169186
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00169186