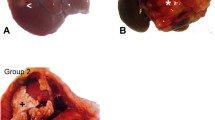
The present study was performed to investigate processes involved in circumvention of the immune system by advanced stages of tumor growth in the liver. The efficacy of Kupffer cells and pit cells against cancer cells was tested in vivo in an experimental model of colon carcinoma metastasis in rat liver. Liver tumors were induced by administration of CC531 colon cancer cells into the vena portae. After 3 weeks, livers were obtained and partly fixed for electron microscopic procedures or frozen in liquid nitrogen for enzyme and immunohistochemistry at the light microscope level. The activation status of Kupffer cells was studied by expression of la-antigen (MHC class II) and by measurement of glucose-6-phosphate dehydrogenase (G6PDH) activity in the cells in situ as a measure of production of reactive oxygen species. Large numbers of Kupffer cells were found in liver parenchyma surrounding colon carcinomas when compared with levels in control livers, but these cells were not activated. Large numbers of activated monocytes and macrophages, cytotoxic T cells but only a few pit cells were found to be recruited to the boundary between liver parenchyma and tumors or their stroma. In those areas where cancer cells invaded liver parenchyma, only newly recruited macrophages and some Kupffer cells were present but few cytotoxic T cells or pit cells were found. The low activation status of Kupffer cells both in terms of production of reactive oxygen species and Ia-antigen expression and the absence of significant numbers of pit cells at tumor sites suggest that Kupffer cells and pit cells do not play a significant role in advanced stages of tumor growth. High levels of prostaglandin E2 were detected in the parenchyma of livers containing tumors and transforming growth factor β was detected in the stroma of the tumors, therefore suggest that cytotoxicity of newly recruited monocytes, macrophages and cytotoxic T cells may be limited in these stages because of local production of these immunosuppressive factors.
Similar content being viewed by others
References
Nicolson GL, 1991, Molecular mechanism of cancer metastasis: tumor and host properties and the role of oncogenes and suppressor genes. Curr Opinion Oncol, 3, 75–92.
Roh MS, Wang L, Oyedeji C, et al. 1990, Human Kupffer cells are cytotoxic against human colon adenocarcinoma. Surgery, 108, 400–5.
Daemen T, Veninga A, Roerdink FH and Scherphof GL, 1986, In vitro activation of rat liver macrophages to a tumoricidal activity by free or liposome-encapsulated muramyl dipeptide. Cancer Res, 46, 4330–5.
Juy D and Chedid L, 1975, Comparison between macrophage activation and enhancement of nonspecific resistance to tumors by mycobacterial immunoadjuvants. Proc Natl Acad Sci USA, 72, 4105–9.
Moras ML, Phillips NC, Bahr GM and Chedid L, 1985, In vitro inhibition of murine B-cell tumor growth by MDP, MDP (D-D) and vaccin is mediated by macrophages. Int J Immunopharmacol, 7, 515–24.
Xu ZL, Bucana CD and Fidler IJ, 1984, In vitro activation of murine Kupffer cells by lymphokines to lyse syngeneic tumor cells. Am J Pathol, 117, 372–9.
Katz S, Grosfeld JL, Gross K, et al. 1984, Impaired bacterial clearance and trapping in the obstructive jaundice. Ann Surg, 199, 14–20.
Decker T, Kiderlen AF and Lohmann-Matthes ML, 1985, Liver macrophages (Kupffer cells) as cytotoxic effector cells in extracellular and intracellular cytotoxicity. Infect Immunol, 50, 358–64.
Bautista AP, Schuler A, Spolarics Z and Spitzer JJ, 1991, Tumor necrosis factor-α stimulates superoxide anion generation by perfused rat liver and Kupffer cells. Am J Physiol, 261, G891–5.
Voth R, Rossol S, Gallati H, et al. 1988, In vivo and in vitro induction of natural killer cells by cloned human tumor necrosis factor. Cancer Immunol Immunother, 27, 128–32.
Cohen SA, Salazar D, Von Muenchhausen W, Werner-Wasik M and Nolan JP, 1985, Natural antitumor defense system of the murine liver. J Leukocyte Biol, 37, 559–69.
Malter M, Friedrich E and Süss R, 1986, Liver as a tumor killing organ: Kupffer cells and natural killers. Cancer Res, 46, 3055–60.
Leung KH, Salazar D, Ip μM and Cohen SA, 1987, Characterization of natural cytotoxic effector cells isolated from rat liver. Nat Immunol Cell Growth Regul, 6, 150–60.
Bouwens L and Wisse E, 1989, Hepatic pit cells have natural cytotoxic (NC) activity against solid tumorderived target cells. In: Wisse E, Knock DL and Decker K, eds. Cells of Hepatic Sinusoids, Vol. 2. Rijswijk: Kupffer Cells Foundation, pp. 215–20.
Wagner HE, Toth CA, Steele GD and Thomas P, 1992, Metastasic potential of human colon cancer cell lines: relationship to cellular differentiation and carcino-embryonic antigen production. Clin Exp Metastasis, 10, 25–31.
Pearson HJ, Anderson J, Chamberlain J and Bell PRF, 1986, The effect of Kupffer cell stimulation or depression on the development of liver metastases in the rat. Cancer Immunol Immunother, 23, 214–16.
Zhang W, Arii S, Sasaoki T, et al. 1993, The role of Kupffer cells in the surveillance of tumor growth in the liver. J Surg Res., 55, 140–6.
Heuff G, Boutkan H, Beelen RHJ, Van Rooijen N, Meyer S and Dijkstra CD, 1993, Kinetics and functional role of Kupffer cells in controlling hepatic metastases. In: Knock DL and Wisse E, eds. Cells of Hepatic Sinusoids, Vol. 4. Leiden: Kupffer Cells Foundation, pp. 500–3.
Saijo N, Okazi A, Beppu Y, et al. 1984, Analysis of metastatic spread and growth of tumor cells in mice with depressed natural killer activity by anti-asialo GMl1 antibody or anticancer agents. J Cancer Res Clin Oncol, 107, 157–63.
Wiltrout RH, Herberman RB, Zhang SR, et al. 1985, Role of organ-associated NK cells in decreased formation of experimental metastases in lung and liver. J Immunol, 134, 4267–75.
Shiratori Y, Tanaka M, Okano K, et al. 1991, Cellular interaction between Kupffer cells and pit cells. In: Wisse E, Knook DL and McCuskey RS, eds. Cells of Hepatic Sinusoids, Vol. 3. Leiden: Kupffer Cells Foundation, pp. 300–5.
Kaneda K, Natsuhara Y, Oka S, Uehara K, Yano I and Wake K, 1989, In vivo defensive role of pit cells in the liver. In: Wisse E, Knook DL and Decker K, eds. Cells of Hepatic Sinusoids, Vol. 2. Rijswijk: Kupffer Cells Foundation, pp. 221–3.
Bouwens L, Charles K, Van Dale P and Wisse E, 1989, Sinusoidal cell reactions associated with colon cancer metastases in rat liver. In: Wisse E, Knook DL and Decker K, eds. Cells of Hepatic Sinusoids, Vol. 2. Rijswijk: Kupffer Cells Foundation, pp. 237–8.
Thomas C, Nijenhuis AM, Timens W, Kuppen PJK, Daemen T and Scherphof GL, 1993, Liver metastases model of colon cancer in the rat: immunohistochemical characterization. Inv Metastasis, 13, 102–12.
Hoedemakers RMJ, Morselt HWM, Scherphof GL and Daemen T, 1995, Heterogeneity in secretory responses of rat liver macrophages of different size. Liver 15, 313–19.
Marquet RL, Westbroek DL and Jeekel J, 1984, Interferon treatment of transplantable rat colon adenocarcinoma: importance of tumor site. Int J Cancer, 33, 689–92.
Jonges GN, Vogels IMC, Bosch KS, Dingemans KP and Van Noorden CJF, 1993, Experimentally induced colon cancer metastases in rat liver increase the proliferation rate and capacity for purine catabolism in liver cells. Histochemistry, 100, 41–51.
Nakane PK, 1968, Simultaneous localization of multiple tissue antigens using the peroxidase-labeled antibody method: a study on pituitary glands of the rat. J Histochem Cytochem, 16, 557–60.
Polak JM and Van Noorden S, 1984, An Introduction to Immunocytochemistry: Current Techniques and Problems. Oxford: Oxford University Press.
Van der Loos CM, Becker AE and Van den Oord JJ, 1993, Practical suggestions for successful immunoenzyme double-staining experiments. Histochem J, 25, 1–13.
Van Noorden CJF and Frederiks WM, 1992, Enzyme Histochemistry: A Laboratory Manual of Current Methods. Oxford: Oxford University Press.
Jonges GN, Vogels IMC and Van Noorden CJF, 1995, Effects of partial hepatectomy, phenobarbital and 3-methylcholanthrene on kinetic parameters of glucose 6-phosphate and phosphogluconate dehydrogenase in situ in periportal and pericentral zones of rat liver lobuli. Biochem Biophys Acta, 1243, 59–64.
Hosemann W, Teutsch HF and Sasse D, 1979, Identification of G6PDH-active sinusoidal cells as Kupffer cells in the rat liver. Cell Tissue Res, 196, 237–47.
Bhatnagar R, Schirmer R, Ernst M and Decker K, 1981, Superoxide release by zymosan-stimulated rat Kupffer cells in vitro. Eur J Biochem, 119, 171–5.
Van Berkel TJC and Koster JF, 1977, Biochemical characteristics of non-parenchymal liver cells. In: Wisse E and Knook DL, eds. Kupffer Cells and Other Sinusoidal Cells. Amsterdam: Elsevier, pp. 299–306.
Spolarics Z, Bautista AP and Spitzer JJ, 1993, Augmented hexose monophosphate shunt activity supports superoxide anion production in Kupffer cells and protects against oxidative injury in hepatic endothelial cells following in vivo endotoxin. In: Knook DL and Wisse E, eds. Cells of Hepatic Sinusoids, Vol. 4. Leiden: Kupffer Cells Foundation, pp. 29–32.
Swezey RR and Epel D, 1986, Regulation of glucose6-phosphate dehydrogenase activity in sea urchin eggs by reversible association with cell structural elements. J Cell Biol, 103, 1509–15.
Swezey RR and Epel D, 1988, Enzyme stimulation upon fertilization is revealed in electrically permeabilized sea urchin eggs. Proc Natl Acad Sci USA, 85, 812–16.
Stanton RC, Seifter JL, Boxer DC, Zimmerman E and Cantley LC, 1991, Rapid release of bound glucose-6-phosphate dehydrogenase by growth factors. J Biol Chem, 266, 12442–8.
Van Noorden CJF and Jonges GN, 1995, In situ analysis of enzyme reactions. Histochem J, 27, 101–18.
Knock DL and Sleyster ECH, 1976, Separation of Kupffer and endothelial cells of the rat liver by centrifugal elutriation. Exp Cell Res, 99, 444–9.
Butcher RG and Van Noorden CJF, 1985, Reaction rate studies of glucose-6-phosphate dehydrogenase activity in sections of rat liver using four tetrazolium salts. Histochem J, 17, 993–1008.
Wilkinson GN, 1961, Statistical estimations in enzyme kinetics. Biochem J, 80, 324–32.
Shoobridge MPK, 1983,A new principle in polychrome staining; a system of automatic staining, complementary to haematoxylin and eosin, and usable as a research tool. Stain Technol, 58, 245–58.
Dingemans KP and Van den Berg Weerman, 1990, Rapid contrasting of extracellular elements in thin sections. Ultrastruct Pathol, 14, 519–27.
Rosenberg SA, 1986, Adoptive immunotherapy of cancer using lymphokine activated killer cells and recombinant interleukin-2. In: DeVita Jr VT, Hellman S and Rosenberg SA, eds. Important Advances in Oncology. Philadelphia: Lippincott.
Rosenberg SA, Lotze MT, Yang JC, et al. 1989, Experience with the use of high-dose interleukin-2 in the treatment of 652 cancer patients. Ann Surg, 210, 474–85.
Stevenson HC, Lacerna LV and Sugarbaker PH, 1988, Ex vivo activation of killer monocytes (AKM) and their application to the treatment of human cancer. J Clin Apheresis, 4, 118–21.
Schaadt M, Pfreundschuh M, Lorscheidt G, Peters KM, Steinmetz T and Diehl V, 1990, Phase II study of recombinant tumor necrosis factor in colorectal carcinoma. J Biol Response Mod 9, 247–50.
Martin M, Chauffert B, Caignard A, Pelletier H, Hammann A and Martin F, 1989, Histoimmunological characterization of the cellular reaction to liver metas tases induced by colon cancer cells in syngeneic rats. Inv Metastasis, 9, 216–30.
Heuff G, Van der Ende MB, Boutkan H, et al. 1993, Macrophage population in different stages of induced hepatic metastases in rats: an immunohistochemical analysis. Scand J Immunol, 38, 10–16.
Smedsrod B, Pertoft H, Eggertsen G and Sundstrom C, 1985, Functional and morphological characterization of cultures of Kupffer cells and liver endothelial cells prepared by means of density separation in Percoll, and selective substrate adherence. Cell Tissue Res, 241, 639–49.
Roitt I, 1991, Essential Immunology. Oxford: Blackwell Scientific Publications.
Daemen T, Veninga A, Regts J and Scherphof GL, 1991, Maintenance of tumoricidal activity and susceptibility to reactivation of subpopulations of rat liver macrophages. J Immunother, 10, 200–6.
Leser HG, Müller-Buscher G and Gerok W, 1986, Murine Kupffer cells (KC) and peritoneal macrophages (PMϕ): differences in tumor cytotoxi city. In: Kirn A, Knock DL and Wisse E, eds. Cells of Hepatic Sinusoids, Vol. 1. Leiden: Kupffer Cells Foundation, pp. 393–7.
Keller R, Keist R, van der Meide PH, Groscurth P, Aguet M and Leist TP, 1987, Induction, maintenance, and reinduction of tumoricidal activity in bone marrow-derived mononuclear phagocytes by Corynebacterium parvum. J Immunol, 138, 2366–71.
Barbera-Guillem E, Canavates ML, Lopez de Tejada I and Vidal-Vanaclocha F, 1988, Influence of host defenses on the hepatic colonization of B16F10 melanoma cells. Clin Exp Metastasis, 6, 153–69.
Taylor DD and Black PH, 1985, Inhibition of macrophage la antigen expression by shed plasma membrane vesicles from metastatic murine melanoma lines. J Natl Cancer Inst, 74, 859–67.
Smorenburg SM, Griffini P, Tiggelman AMBC, Moorman AFM, Boers W and Van Noorden CJF, 1996, α2-Macroglobulin is mainly produced by cancer cells and not by hepatocytes in rats with colon carcinoma metastases in liver. Hepatology, 23, 560–70.
Ertel BW, Morrison MH, Ayala A and Chaudry IH, 1991, Chloroquine attenuates hemorrhagic shockinduced suppression of Kupffer cell antigen presentation and major histocompatibility complex class II antigen expression through blockade of tumor necrosis factor and prostaglandin release. Blood, 78, 1781–8.
Baxevanis CN, Reclos GJ, Gritzapis AD, et al. 1993, Elevated prostaglandin E2 production by monocytes is responsible for the depressed levels of natural killer and lymphokine-activated killer cell function in patients with breast cancer. Cancer, 72, 491–501.
Droller MJ, Perlmann P and Schneider MU, 1978, Enhancement of natural and antibody-dependent lymphocyte cytotoxicity by drugs which inhibit prostaglandin production by tumor target cells. Cell Immunol, 39, 154–64.
Taffet SM and Russell SW, 1981, Macrophage-mediated tumor cell killing: regulation of expression of cytolytic activity by prostaglandin E. J Immunol, 126, 424–7.
Pelus LM and Bockman RS, 1979, Increased prostaglandin synthesis by macrophages from tumorbearing mice. J Immunol, 123, 2118–25.
Maxwell WJ, Kelleher D, Keating JJ, et al. 1990, Enhanced secretion of prostaglandin E2 by tissue-fixed macrophages in colonic carcinoma. Digestion, 47, 160–6.
Oka M, Inaba A, Uchiyama T, et al. 1994, Prostaglandin E2 levels and lymphocyte subsets in portal venous drainage of colorectal cancers. Am J Surg, 167, 264–7.
Botha JH, Robinson KM, Ramchurren N, Reddi K and Norman RJ, 1986, Human esophageal carcinoma cell lines: prostaglandin production, biological prop erties and behaviour in nude mice. J Natl Cancer Inst, 76, 1053–6.
Parker SG, Brouwer A, Hendriks HFJ and Horan MA, 1993, Stimulation of Kupffer cells from young and old rats by endotoxin: production of eicosanoids and cytokines and induction of early response proteins. In: Knock DL and Wisse E, eds. Cells of Hepatic Sinusoids, Vol. 4. Leiden: Kupffer Cells Foundation, pp. 21–5.
Wu JZ, Ogle CK, Ogle JD and Alexander JW, 1993, A comparison of hepatic, splenic, peritoneal and alveolar macrophages with respect to PGE2 TXB2 production and ADCC function. Prostagland Leukotrienes Essential Fatty Acids, 48, 149–53.
Okumura Y, Ishibashi H, Shirahama M, et al. 1987, Kupffer cells modulate natural killer cell activity in vitro by producing prostaglandins. Cell Immunol, 107, 89–98.
Grossi CE, Zicca A, Cadoni A, Mingari MC, Moretta A and Moretta L, 1983, Ultrastructural characteristics of human T cell clones with various cytolytic activities. Eur J Immunol, 13, 670–7.
Bouwens L, Monden K, Vanderkerken K, Van den Berg K and Wisse E, 1993, Effects of colon carcinoma tumors in rat liver on the composition and cytotoxic activity of hepatic pit cells. In: Knook DL and Wisse E, eds. Cells of Hepatic Sinusoids, Vol. 4. Leiden: Kupffer Cells Foundation, pp. 548–50.
Lala PK, Sauter V, Libenson H and Parhar RS, 1985, Changes in the host natural killer cell population in mice during tumor development. Cell Immunol, 93, 250–64.
Nolibé D and Poupon MF, 1986, Enhancement of pulmonary metastases induced by decreased lung natural killer cell activity. J Natl Cancer Inst, 77, 99–103.
Winnock M, Garcia-Barcina M, Huet S, et al. 1993, Functional characterization of liver-associated lymphocytes of patients with liver metastasis. Gastroenterology, 105, 1152–8.
Steinhauer E, Doyle AT, Reed J and Kadish AS, 1982, Defective natural cytotoxicity in patients with cancer: normal number of effector cells but decreased re cycling capacity in patients with advanced disease. J Immunol, 129, 2255–9.
Tartter PI, Steinberg B, Barron DM and Martinelli G, 1987, The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch Surg, 122, 1264–8.
Bachem MG, Meyer D, Melchior R, Sell KM and Gressner AM, 1992, Activation of rat liver perisinusoidal lipocytes by transforming growth factors derived from myofibroblast-like cells. J Clin Invest, 89, 19–27.
Lucas C, Bald LN, Fendly BM, et al. 1990, The autocrine production of transforming growth factorβ1 during lymphocyte activation: a study with a monoclonal antibody-based ELISA. J Immunol, 145, 1415–22.
Assoian RK, Fleurdelys BE, Stevenson HC, et al. 1987, Expression and secretion of type β transforming growth factor by activated human macrophages. Proc Natl Acad Sci USA, 84, 6020–4.
Wahl SM, Hunt DA, Wakefield LM, et al. 1987, Transforming growth factor-beta (TGFP) induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA, 84, 5788–94.
Rook AH, Kehrl JH, Wakefield LM, et al. 1986, Effects of transforming growth factor β on the function of natural killer cells: depressed cytolytic activity and blunting of interferon responsiveness. J Immunol, 136, 3916–20.
Malygin AM, Meri S and Timonen T, 1993, Regulation of natural killer cell activity by transforming growth factor-β and prostaglandin E2. Scand J Immunol, 37, 71–6.
Dijkstra CD, Döpp EA, Joling P and Kraal G, 1984, The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rats recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology, 54, 589–99.
McMaster WR and Williams AF, 1979, Identification of la glycoproteins in rat thymus and purification from rat spleen. Eur J Immunol, 9, 426–33.
Bouwens L and Wisse E, 1992, Pit cells in the liver. Liver, 12, 3–9.
Van den Brink MRM, Palomba ML, Basse PH and Hiserodt JC, 1991, In situ localization of 3.2.3+ natural killer cells in tissue from normal and tumor-bearing rats. Cancer Res, 51, 4931–6.
Caldero J, Campo E, Vinas J and Cardesa A, 1989, Lectin-binding sites in neoplastic and non-neoplastic colonic mucosa of 1,2-dimethylhydrazine-treated rats. Lab Invest, 61, 670–6.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Griffini, P., Smorenburg, S.M., Vogels, I.M.C. et al. Kupffer cells and pit cells are not effective in the defense against experimentally induced colon carcinoma metastasis in rat liver. Clin Exp Metast 14, 367–380 (1996). https://doi.org/10.1007/BF00123396
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00123396