Abstract
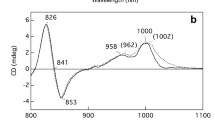
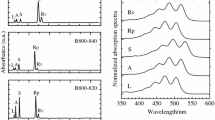
The modification effects on the absorption and cirular dichroic (CD) spectra of the isolated B800-860 antenna complex of Rhodocyclus tenuis by a number of proteolytic enzymes were investigated. The chymotrypsin modifications of the B800-860 complex led to an about 40% decrease of the 860-nm band and a blue-shift to 841 nm. The biphasic CD signal related to the B860 BChl disappeared and a new double CD signal with a zero-crossing point at 842 nm appeared. These absorption and CD spectral changes suggested that a B800-841 complex resulted after chymotrypsin digestion. The polypeptide components of the chymotrypsin-modified B800-860 complex were separated by reverse-phase chromatography, and their amino acid sequences determined by protein sequencing and mass spectrometry. Sequence analyses showed that the C-terminal 25 residues of the B800-860-α polypeptide and the C-terminal 8 residues of the B800-860-β polypeptide were cleaved by chymotrypsin, and the remaining α, β polypeptide fragments apparently form the structural basis for the newly-formed B800-841 complex. No significant spectral change was observed from exposing the isolated B800-860 complex to trypsin, carboxypeptidase A and the combination of carboxypeptidase A and carboxypeptidase B. Short-term proteinase K incubation of the B800-860 complex of Rc. tenuis led to a preferential decrease of the 860-nm absorbance band and its related CD signals, as compared to the 800-nm absorbance and CD bands, suggesting that the C-terminal portions of the antenna polypeptides are possibly exposed to the exterior of the B800-860 complex micelles. Whereas, long-term proteinase K digestion resulted in the spectral collapse of the B800-860 complex and the release of free BChls. Our proteolysis experiments support the hypothesis that the C-terminal portions of the antenna polypeptides play a key role in the redshift and strong molar extinction of the Qy band of the B850 BChls.
Similar content being viewed by others
Abbreviations
- B800-860:
-
light-harvesting complex with the absorption maxima (Qy) at 800 nm and 860 nm
- B800-860-α:
-
β-α,β polypeptide of the B800-860 complex
- CD:
-
circular dichroism
- Deriphat-160:
-
disodium Nlauryl-β-iminodipropionate
- FT:
-
Fourier transform
- LH:
-
light-harvesting
- near-IR:
-
near infra-red
- OG:
-
n-Octyl-β-glucoside
- PTH:
-
phenylthiohydantoin
- Rb. :
-
Rhodobacter
- Rc. :
-
Rhodocyclus
- Rp. :
-
Rhodopseudomonas
- Rsp :
-
Rhodospirillum
- DSM:
-
Deutsche Sammlung für Mikroorganismen
References
Babst M, Albrecht H, Wegmann I, Brunisholz RA and Zuber H (1991) Single amino acid substitutions in the B870-α and β light-harvesting polypeptides of Rhodobacter capsulatus: Structural and spectral effects. Eur J Biochem 202: 277–284.
Brunisholz RA and Zuber H (1988) Primary structure analyses of bacterial antnna polypeptides: Correlation of aromatic amino acids with spectral properties, structural similarities with reaction center polypeptides. In: Scheer H and Schneider S (eds) Photosynthetic Light-Harvesting System, pp 103–114. Walter de Gruyter, Berlin, New York.
Brunisholz RA and Zuber H (1992) Structure, function and organization of antenna polypeptides and antenna complexes from the three families of Rhodospirillaneae. J Photochem Photobiol B: Biol 15: 113–140.
Brunisholz RA and Zuber H (1993) Spectral modifications of bacterial antenna complexes by limited proteolysis. Photochem Photobiol 57: 6–12.
Brunisholz RA, Suter F and Zuber H (1994) Structural and spectral characterization of the antanna complexes of Rhodocyclus gelatinosus: Indications of a hairpin-like-arranged antenna apoprotein with an unusually high alanine content. Eur J Biochem 222: 667–675.
DeBoer WE (1969) On ultrastructures in Rhodopseudomonas gelatinosa and Rhodospirillum tenue. Antonic van Leeuwenhoek. J Microbiol Serol 35: 241–242.
Fowler GJS, Visschers RW, Grief GG, vanGrondelle R and Hunter CN (1992) Genetically modified photosynthetic antenna complexes with blueshifted absorbance bands. Nature 355: 848–850.
Fowler GJS, Sockalingum GD, Robert B and Hunter CN (1994) Blue shifts in bacteriochlorophyll absorbance correlate with changed hydrogen bonding patterns in light-harvesting 2 mutants of Rhodobacter sphaeroides with alterations at α-Tyr-44 and α-Tyr-45. Biochem J 299: 695–700.
Hu Q (1994) Isolation and structural and spectral characterization of the light-harvesting pigment-protein complexes and their polypeptides from the purple bacterium Rhodocyclus tenuis. PhD Thesis, ETH No. 10846, Zurich, Switzerland.
Hu Q, Brunisholz RA, Frank G and Zuber H (1996) The antenna complexes of the purple non-sulfur photosynthetic bacterium Rhodocyclus tenuis: structural and spectral characterization. Eur J Biochem 238: 381–390.
Imhoff JF, Trüper HG and Pfennig N (1984) Rearrangement of the species and the genera of the phototrophic ‘purple non-sulfur bacteria’. Int J Sys Bacteriol 34: 340–343.
Koepke J, Hu X, Muenke C, Schulten K, and Michel H (1996) The crystal structure of the light-harvesting complex II (B800–850) from Rhodospirillum molischianum. Structure 4: 581–597.
McDermott G, Prince SM, Freer AA, Hawthornthwaite-Lawless AM, Papiz MZ, Cogdell RJ and Isaacs NW (1995) Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature 374: 517–521.
Pfennig N (1969) Rhodospirillum tenue sp. n., a new species of the purple nonsufur bacteria. J Bacteriol 99: 619–620.
Sturgis JN, Jirsakova V, Reiss-Husson F, Cogdell RJ and Robert B (1995) Structure and properties of the bacteriochlorophyll binding site in peripheral light-harvesting complexes of purple bacteria. Biochemstry 34: 517–523.
vanGrondelle R, Dekker JP, Gillbro T and Sundstrom V (1994) Energy transfer and trapping in photosynthesis. Biochim Biophys Acta 1187: 1–65.
Zuber H and Brunisholz RA (1991) Structure and function of antenna polypeptides and chlorophyll-protein complexes: Principles and variability. In: Scheer H (ed) Chlorophylls, pp 627–703. CRC Press, Boca Raton, FL.
Zuber H (1993) Structural features of photosynthetic light-harvesting systems. In: The Deidenhofer J and Norris R (eds) Photosynthetic Reaction Center, Vol I, pp 43–100. Academic Press, San Diego, CA.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hu, O., Brunisholz, R.A. & Zuber, H. Proteolytic modifications of the B800-860 complex of the photosynthetic bacterium Rhodocyclus tenuis: Structural and spectral effects. Photosynth Res 50, 223–232 (1996). https://doi.org/10.1007/BF00033121
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00033121