Abstract
Background: Hyperglycaemia is associated with serious complications, significant morbidity and death. Despite the availability of a wide range of therapeutic options, many patients with diabetes mellitus fail to achieve or maintain recommended glycaemic goals. Ipragliflozin (ASP1941) is a novel, selective inhibitor of the sodium-dependent glucose co-transporter 2, which is highly expressed in the proximal tubules of the kidneys. It suppresses renal glucose reabsorption and increases urinary glucose excretion (UGE), potentially providing an insulin-independent treatment option for type 2 diabetes.
Methods: This multiple ascending-dose study assessed the safety, tolerability, pharmacokinetics and pharmacodynamics of ipragliflozin in healthy subjects after single doses and multiple once-daily doses for 10 days (dose levels: 5–600 mg).
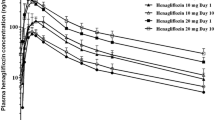
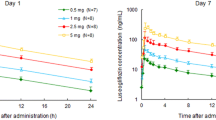
Results: Ipragliflozin was well tolerated following single and multiple once-daily oral dosing. Ipragliflozin was rapidly absorbed with a median time to reach the maximum plasma concentration of 1.3 hours after the last dose. The area under the plasma concentration-time curve increased proportionally with increasing dose. The mean elimination half-life was 12 hours following the last dose. Ipragliflozin dose dependently increased UGE up to a maximum of approximately 59 g (327 mmol) of glucose excreted over 24 hours following multiple doses, without affecting plasma glucose levels in healthy subjects.
Conclusion: Administration of ipragliflozin was well tolerated and resulted in a rapid, dose-dependent increase in glucosuria. Pharmacodynamic and pharmacokinetic data suggest that ipragliflozin is suitable for prolonged once-daily oral treatment.
Trial Registration: ClinicalTrials.gov Identifier: NCT01288898.









Similar content being viewed by others
References
Leahy JL. Pathogenesis of type 2 diabetes mellitus. Arch Med Res 2005; 36: 197–209
Moss SE, Klein R, Klein BE, et al. The association of glycemia and cause-specific mortality in a diabetic population. Arch Intern Med 1994; 154: 2473–9
Klein R. Hyperglycemia and microvascular and macro-vascular disease in diabetes. Diabetes Care 1995; 18: 258–68
Nathan DM, Buse JB, Davidson MB, et al., American Diabetes Association; European Association for Study of Diabetes. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy. A consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2009; 32: 193–203
Saydah SH, Fradkin J, Cowie CC. Poor control of risk factors for vascular disease among adults with previously diagnosed diabetes. JAMA 2004; 291: 335–42
Nair S, Wilding JP. Sodium glucose cotransporter 2 inhibitors as a new treatment for diabetes mellitus. J Clin Endocrinol Metab 2010; 95: 34–42
Ismail-Beigi F, Craven T, Banerji MA, et al. Effect of intensive treatment of hyperglycaemia on microvascular outcomes in type 2 diabetes: an analysis of the ACCORD randomised trial. Lancet 2010; 376: 419–30
Marsenic O. Glucose control by the kidney: an emerging target in diabetes. Am J Kidney Dis 2009; 53: 875–83
Wright EM. Renal Na(+)-glucose cotransporters. Am J Physiol Renal Physiol 2001; 280: F10–8
Neumiller JJ, White Jr JR, Campbell RK. Sodium-glucose co-transport inhibitors: progress and therapeutic potential in type 2 diabetes mellitus. Drugs 2010; 70: 377–85
Jabbour SA, Goldstein BJ. Sodium glucose co-transporter 2 inhibitors: blocking renal tubular reabsorption of glucose to improve glycaemic control in patients with diabetes. Int J Clin Pract 2008; 62: 1279–84
Vallon V, Platt KA, Cunard R, et al. SGLT2 mediates glucose reabsorption in the early proximal tubule. J Am Soc Nephrol 2011; 22: 104–12
Turk E, Zabel B, Mundlos S, et al. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 1991; 350: 354–6
Santer R, Kinner M, Lassen CL, et al. Molecular analysis of the SGLT2 gene in patients with renal glucosuria. J Am Soc Nephrol 2003; 14: 2873–82
Vestri S, Okamoto MM, de Freitas HS, et al. Changes in sodium or glucose filtration rate modulate expression of glucose transporters in renal proximal tubular cells of rat. J Membr Biol 2001; 182: 105–12
Rahmoune H, Thompson PW, Ward JM, et al. Glucose transporters in human renal proximal tubular cells isolated from the urine of patients with non-insulin-dependent diabetes. Diabetes 2005; 54: 3427–34
Freitas HS, Anh e GF, Melo KF, et al. Na(+)-glucose transporter-2 messenger ribonucleic acid expression in kidney of diabetic rats correlates with glycemic levels: involvement of hepatocyte nuclear factor-1alpha expression and activity. Endocrinology 2008; 149: 717–4
List JF, Woo V, Morales E, et al. Sodium-glucose cotransport inhibition with dapagliflozin in type 2 diabetes. Diabetes Care 2009; 32: 650–7
Patel AK, Fonseca V. Turning glucosuria into a therapy: efficacy and safety with SGLT2 inhibitors. Curr Diab Rep 2010; 10: 101–7
Kurosaki E, Tahara A, Yokono M, et al. In vitro and in vivo pharmacological properties of ASP1941, a novel, potent and selective SGLT2 inhibitor [abstract no. 0570-P]. American Diabetes Association, 70th Scientific Sessions; 2010 June 25–29; Orlando (FL)
Takasu T, Tahara A, Yokono M, et al. ASP1941, a novel, potent and selective SGLT2 inhibitor, improves hemoglobin A1c and symptoms of diabetes in animal models [abstract no. 0562-P]. American Diabetes Association, 70th Scientific Sessions; 2010 June 25–29; Orlando (FL)
Data on file. Internal Report 1941-ME-0040. Identification of ASP1941 metabolites in humans, 12 March 2010. Astellas Pharma Inc., Kashima, Osaka
Data on file. Internal Report 1941-PH-0029. Inhibitory activities of human metabolites of ASP1941 on cellular uptake of methyl-α-D-glucopyranoside (AMG) in human Na+-glucose cotransporter (SGLT)-expressing cells, 12 April 2010. Astellas Pharma Inc., Tsukuba, Ibaraki
Data on file. Internal report 1941-ME-0045. Correlation of ASP1941 metabolic activity with UGT enzyme activities in human liver microsomes, 29 March 2010. Astellas Pharma Inc., Kashima, Osaka
Data on file. Internal Report 1941-ME-0055. Inhibitory effects of ASP1941 on the activity of UDP-glucuronosyltransferases using human liver microsomes, 09 December 2010. XenoTech, LLC, Lenexa (KS)
Data on file. Internal Report 1941-ME-0039. Time-dependent inhibitory effects of ASP1941 on the activity of CYP isozymes using human liver microsomes, 16 October 2009. Sekisui Medical Co., Ltd, Muramatsu, Ibaraki
Tassi C, Mancuso F, Feligioni L, et al. Expression modes of urinary N-acetyl-beta-D-glucosaminidase in patients with chronic renal insufficiency. Clin Chim Acta 2004; 346: 129–33
Lisowska-Myjak B. Serum and urinary biomarkers of acute kidney injury. Blood Purif 2010; 29: 357–65
Data on file. Internal Report 1941-ME-0009. Validation of a LC-MS/MS method for the determination of ASP1941 in human plasma, 04 December 2007. Astellas Pharma Europe B.V., Leiderdorp
Data on file. Internal Report 1941-ME-0010. Validation of a LC-MS/MS method for the determination of ASP1941 in human urine, 04 December 2007. Astellas Pharma Europe B.V., Leiderdorp
Bailey CJ, Gross JL, Pieters A, et al. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet 2010; 375: 2223–33
Sobel JD. Candidal vulvovaginitis. Clin Obstet Gynecol 1993; 36: 153–65
Geerlings SE, Stolk RP, Camps MJ, et al., Diabetes Women Asymptomatic Bacteriuria Utrecht Study Group. Risk factors for symptomatic urinary tract infection in women with diabetes. Diabetes Care 2000; 23: 1737–41
Meng W, Ellsworth BA, Nirschl AA, et al. Discovery of dapagliflozin: a potent, selective renal sodium-dependent glucose cotransporter 2 (SGLT2) inhibitor for the treatment of type 2 diabetes. J Med Chem 2008; 51(5): 1145–9
Komoroski B, Vachharajani N, Boulton D, et al. Dapagliflozin, a novel SGLT2 inhibitor, induces dose-dependent glucosuria in healthy subjects. Clin Pharmacol Ther 2009; 85: 520–6
Schwartz S, Klasen S, Kowalski D, et al. ASP1941, a novel and selective inhibitor of sodium-glucose co-transporter 2 (SGLT2), reduces fasting plasma glucose in patients with type 2 diabetes mellitus [abstract no. 0566-P]. American Diabetes Association, 70th Scientific Sessions; 2010 June 25–29; Orlando (FL)
Komoroski B, Vachharajani N, Feng Y, et al. Dapagliflozin, a novel, selective SGLT2 inhibitor, improved glycemic control over 2 weeks in patients with type 2 diabetes mellitus. Clin Pharmacol Ther 2009; 85: 513–9
Kapur AR, O’Connor-Semmes RL, Hussey EK, et al. First human dose escalation study with remogliflozin etabonate (RE) in healthy subjects and in subjects with type 2 diabetes mellitus [abstract no. 509-P]. American Diabetes Association, 69th Scientific Sessions; 2009 June 5–9; New Orleans (LA)
Boyle PJ, Zrebiec J. Physiological and behavioral aspects of glycemic control and hypoglycemia in diabetes. South Med J 2007; 100: 175–82
Landau BR, Wahren J, Chandramouli V, et al. Contributions of gluconeogenesis to glucose production in the fasted state. J Clin Invest 1996; 98: 378–85
Acknowledgements
This study was funded by Astellas Pharma, Europe BV, Leiderdorp, the Netherlands. All authors are employees of Astellas Pharma. Ipragliflozin is under development by Astellas Pharma Inc. and Kotobuki Pharmaceutical Co., Ltd. The authors thank Sandra Mendes from Excerpta Medica, who provided writing/editorial assistance for this work, which was supported by Astellas.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Veltkamp, S.A., Kadokura, T., Krauwinkel, W.J.J. et al. Effect of Ipragliflozin (ASP1941), a Novel Selective Sodium-Dependent Glucose Co-Transporter 2 Inhibitor, on Urinary Glucose Excretion in Healthy Subjects. Clin. Drug Investig. 31, 839–851 (2011). https://doi.org/10.1007/BF03256922
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF03256922