Abstract
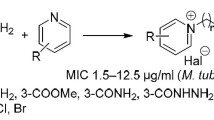
A series of 124 basic ethyl esters of alkoxy-substituted phenylcarbamic acids with the alkoxy group in position 2, 3 or 4 on the phenyl ring, and basic substitutents attached to the ethyl moiety in position 2, were evaluated forin vitro antimycobacterial activity against strains ofMycobacterium tuberculosis, Mycobacterium kansasii andMycobacterium avium. In vitro antimycobacterial activity becomes higher with increasing hydrophobic properties of the alkoxy groups. Thep- andm-substituted derivatives were more active than theo-substituted ones. Direct relationship between the structure of the basic substituents and the activity was not found.
Similar content being viewed by others
References
Čižmárik J., Borovanský A., Švec P.: Studies of local anæsthetics. LVII. Perhydroazepinyl esters of alkoxyphenyl carbamic acids. (In Slovak)Českoslov.Farm. 25, 118–121 (1976a).
Čižmárik J., Borovanský A., Švec P.: Study of local anæsthetics. XII. Piperidinoethylesters of alkoxyphenylcarbamic acids.Acta Fac.Pharm.Univ.Comeniae 29, 53–80 (1976b).
Čižmárik J., Mitošinková M., Borovanský A., Švec P.: Dialkylaminoathylesters der Alkoxyphenylcarbamidsäuren.Pharmazie 33, 509–512 (1978).
Čižmárik J., Šveinochová M., Schwartz E.: Tuberkulostatische Wirkung der Piperidinoethylesters der Alkoxyphenylcarbamidsäurenin vitro.Pharmazie 41, 744–745 (1986).
Čižmárik J., Borovanský A., Tŭmová I.: Untersuchungen an Localanesthetica. Teil 91. Morpholinoethylesters der Alkoxyphenylcarbamidsäuren.Pharmazie 42, 702–703 (1987).
Čižmárik J., Polášek E., Švec P., Rančanská E.: Studies of local anesthetics. CX. Preparation, activity and partition coefficients of pyrrolidinoesters of 2-, 3- and 4-alkoxy-substituted phenylcarbamic acids.Českoslov.Farm. 42, 88–91 (1993).
Fujita T., Ban T.: Structure-activity study of phenethylamines as substrates of biosynthetic enzymes of sympathetic enzymes of sympathetic transmitters.J.Med.Chem. 14, 148–152 (1971).
Klimešová V., Svoboda M., Waisser K., Kaustová J., Buchta V., Králová K.: Synthesis of 2-benzylthiopyridine-4-carbothioamide derivatives and their antimycobacterial, antifungal and photosynthesis-inhibiting activity.Eur.J.Med.Chem. 34, 433–440 (1999).
Klimešová V., Svoboda M., Waisser K., Pour M., Kaustová J.: New pyridine derivatives as potential antimicrobial agents.Farmaco 54, 666–672 (1999).
Kubicová L., Waisser K., Kuneš J., Kralová K., Odlerová Ž., Šlosárek M., Janota J., Svoboda Z.: Synthesis ofN,N′-diarylalkyldiamines and their antimycobacterial and antialgal activity.Molecules 5, 714–726 (2000).
Kuneš J., Bažant J., Pour M., Waisser K., Šlosárek M., Janota J.: Quinazoline derivatives with antitubercular activity.Farmaco 55, 725–729 (2000).
Kuneš J., Špulák M., Waisser K., Šlosárek M., Janota J.: Quinoxaline derivatives as potential antitubercular agents.Pharmazie 55, 858–859 (2000).
Waisser K.: Lipophility of antituberculotics.N-Alkyl-2-furamides. (In Czech)Českoslov.Farm. 38, 385–387 (1989a).
Waisser K.: Antituberculcus agents. 51. On the problem of optimal lipophilicity of antituberculous agents — 5-nitro-2-alkoxymethylfuranes.Folia Pharm.Univ.Carol. 15, 67–71 (1989b).
Waisser K., Hladŭvková J., Gregor J., Rada T., Kubicová L., Klimešová V., Kaustová J.: Relationships between the chemical structure of antimycobacterial substances and their activity against atypical strains. Part 14: 3-Aryl-6,8-dihalogeno-2H-1,3-benzoxazine-2,4(3H)-diones.Arch.Pharm. 331, 3–6 (1998).
Waisser K., Kubicová L., Kaustová J., Bartsch H., Ekker T., Hanuš V.: Some antimycobacterial thiolactams.Sci.Pharm. 67, 123–127 (1999a).
Waisser K., Macháček M., Dostál H., Gregor J., Kubicová L., Klimešová V., Kuneš J., Palát K. Jr.,Hladůvková J., Kaustová J., Möllmann U.: Relationships between the chemical structure of substances and their antimycobacterial activity against atypical strains. Part 18. 3-Phenyl-2,4-benzoxazine-2,4(3H)-diones and isosteric 3-phenylquinazoline-2,4(1H,3H)-diones.Collect.Czech.Chem.Commun. 64, 1902–1924 (1999b).
Waisser K., Gregor J., Kubicová L., Klimešová V., Kuneš J., Macháček M., Kaustová J.: New groups of antimycobacterial agents: 6-chloro-3-phenyl-4-thioxo-2H-1,3-benzoxazine-2(3H)-ones and 6-chloro-3-phenyl-2H-1,3-benzoxazine-2,4(3H)-dithiones.Eur.J.Med.Chem. 35, 733–741 (2000).
Waisser K., Hladůvková J., Holý P., Macháček M., Karajannis P., Kubicová L., Klimešová V., Kuneš J., Kaustová J.: 2H-1,3-Benzoxazine-2,4(3H)-clones substituted in position 6 as antimycobacterial agents.Chem.Papers 55, 323–334 (2001a).
Waisser K., Hladŭvková J., Kuneš J., Kubicová L., Klimešová V., Karajannis P., Kaustová J.: Synthesis and antimycobacterial activity of salicylanilides substituted in position 5.Chem.Papers 55, 121–129 (2001b).
Waisser K., Čižmárik J., Dražková K., Kaustová J.: Antimycobacterial effects of pyrrolidinoethylesters of alkoxysubstituted phenylcarbamic acids.Česk.Stov.Farm. 51, 140–144 (2002a).
Waisser K., Kubicová L., Buchta V., Kubanová P., Bajerová K., Jirásková L., Bednařík O., Bureš O., Holý P.:In vitro antifungal activity of 3-phenyl-2H-benzoxazine-2,4(3H)-diones.Folia Microbiol. 47, 488–492 (2002b).
Author information
Authors and Affiliations
Additional information
Dedicated to Prof. RNDr. Bohumil Sikyta, DSc., on the occasion of his 70th birthday
This work was supported by theMinistry of Education, Youth and Sports of the Czech Republic (project no. MSM 111 600 001) and by the projectKontakt (78/ČR, 237/SR).
Rights and permissions
About this article
Cite this article
Waisser, K., Dražková, K., Čižmárik, J. et al. Antimycobacterial activity of basic ethyl esters of alkoxy-substituted phenylcarbamic acids. Folia Microbiol 48, 45–50 (2003). https://doi.org/10.1007/BF02931274
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02931274