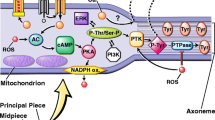
Abstract
The excessive generation of reactive oxygen species (ROS) by abnormal spermatozoa and contaminating leukocytes has been defined as one of the few etiologies for male infertility. Administration of antioxidants in patients with ‘male factor’ infertility has begun to attract considerable interest. The main difficulty of such an approach is our incomplete understanding of the role of free radicals in normal and abnormal sperm function leading to male infertility. Mammalian spermatozoa membranes are very sensitive to free radical induced damage mediated by lipid peroxidation, as they are rich in polyunsaturated fatty acids. Limited endogenous mechanisms exist to reverse these damages. ROS attacks the fluidity of the sperm plasma membrane and the integrity of DNA in the sperm nucleus. ROS induced DNA damage accelerate the germ cell apoptosis. Unfortunately spermatozoa are unable to repair the damage induced by excessive ROS as they lack the cytoplasmic enzymes required to accomplish the repair. Assessment of such oxidative stress status (OSS) may help in the medical treatment. Treatment strategies must be directed toward lowering of ROS levels to keep only a small amount necessary to maintain normal cell function.
Similar content being viewed by others
References
Hull, M., Glazener, C., Kelly, N., Conway, D., Foster, P., Hunton, R., Coulson, C., Lambert, P., Watt, E. and Desai, K. (1985) Population study of causes, treatment and outcome of infertility. Br Med J. 291, 1693–1697.
Maneesh, M., Jayalekshmi, H., Dutta, S., Chakrabarti, A. and Vasudevan, D. M. (2005) Effect of chronic ethanol administration on testicular antioxidant system and steroidogenic enzymes in rats. Ind. J. of Exp. Biol. 43, 445–449.
Maneesh, M., Jayalekshmi, H., Singh, T. A. and Chakrabarti, A. (2006) Impaired hypothalamic pituitary gonadal axis function in men with diabetes mellitus. Ind. J. of Clin. Biochem. 21 (1), 165–168.
Koppenol, W., Moreno, J., Pryor, W., Ischiropoulos, H. & Beckman J. S. (1992) Peroxynitrite, a cloaked oxidant formed by nitric oxide and superoxide. Chemical Res. in Toxicol. 5, 834–842.
Sikka, S. C. (1996) Oxidative stress and role of antioxidants in normal and abnormal sperm function. Front. Biosci. 1, 78–86.
Alvarez J. G. and Storey, B. T. (1995) Differential incorporation of fatty acids into and peroxidative loss of fatty acids from phospholipids of human spermatozoa. Mol. Reprod. Dev. 42, 334–346.
Armstrong, J. S., Rajasekaran, M., Hellstrom, W. J. and Sikka, S. C. (1998) Antioxidant potential of human serum albumin: role in the recovery of high quality human spermatozoa for assisted reproductive technology. J. Androl. 19, 412–419.
Alvarez, J. G., Touchstone, J. C., Blasco, L. and Storey, B. T. (1987) Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa. Superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 8, 338–348.
Aitken, R. J., Paterson, M., Fisher, H., Buckingham, D. W. and van Duin, M. (1995) Redox regulation of tyrosine phosphorylation in human spermatozoa and its role in the control of human sperm function. J. Cell. Sci. 8, 2017–2025.
Calvin, H. I., Cooper, G. W. and Wallace, E. W. (1981) Evidence that selenium in rat sperm is associated with a cysteine rich structural proteins of the mitochondrial capsule. Gamete Res. 4, 139–145
Lenzi, A., Picardo, M., Gandini, L., Lombardo, F., Terminali, O., Passi, S. and Dondero, F. (1994) Glutathione treatment of dyspermia: effect on the lipoperoxidation process. Hum. Reprod. 9, 2044–2050
Irvine, D. S. (1996) Glutathione as a treatment for male infertility. Reviews of Reprod. 1, 6–12
Wefers, H. and Sies, H (1988) The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem. 174, 353–357.
Maneesh, M., Jayalekshmi, H., Dutta, S., Chakrabarti, A. and Vasudevan, D. M. (2005) Experimental therapeutical intervention with ascorbic acid in ethanol induced testicular injuries in rats. Ind. J. of Exp. Biol. 43, 172–176.
Buettner, G. R. (1993) The pecking order of free radicals and antioxidants, lipid peroxidation, alpha-tocopherol and ascorbate. Arch. Biochem. Biophys. 300, 535–543.
Sies, H. (1993) Strategies of antioxidant defence. Eur. J. Biochem. 215, 213–219.
Iwasaki, A. and Gagnon, C. (1992) Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil. Steril. 57, 409–416.
Sikka, S. C., Rajasekaran, M. and Hellstrom, H. J. (1995) Role of oxidative stress and antioxidants in male infertility. J. Androl. 16, 464–468.
Joyce, D. A. (1987) Oxygen radicals in disease. Adverse Drug Reaction Bull. 127, 476–479.
Sharma, R. K. and Agarwal, A. (1996) Role of reactive oxygen species in male infertility [review]. Urology. 48, 835–850.
Rajasekaran, M., Hellstrom, W. J. and Sikka, S. C. (1996) Quantitative assessment of cytokines (GRO-a and IL-10) in human seminal plasma during genitourinary inflammation. Am. J. Reprod. Immun. 36.
Thomas, J., Fishel, S. B., Hall, J. A., Green, S., Newton, T. A. and Thornton, S. J. (1997) Increased polymorphonuclear granulocytes in seminal plasma in relation to sperm morphology. Hum. Reprod. 12, 2418–2421.
Wolff, H. (1995) The biologic significance of white blood cells in semen. Fertil. Steril. 63, 1143–1147.
Tomlinson, M. J., Barrat, G. L. R. and Cooke, I. D. (1993) Prospective study of leukocytes and leukocyte subpopulations in semen suggests that they are not a cause of male infertility. Fertil. Steril. 60, 1069–1075.
Kessopoulou, E., Tomlinson, M. J., Barratt, C. L., Bolton, A. E. and Cooke, I. D. (1992) Origin of reactive oxygen species in human semen: spermatozoa or leucocytes? J. Reprod. Fertil. 94, 463–470.
Krausz, C., Mills, C., Rogers, S., Tan, S. L. and Aitken, R. J. (1994) Stimulation of oxidant generation by human sperm suspensions using phorbol esters and formyl peptides: relationships with motility and fertilizationin vitro. Fertil. Steril. 62, 599–605.
Iwasaki, A. and Gagnon, C. (1992) Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil. Steril. 57, 409–416.
Aitken, R. J. and Clarkson, J. S. (1987) Cellular basis of defective sperm function and its association with the genesis of reactive oxygen species by human spermatozoa. J. Reprod. Fertil. 81, 459–469.
Ollero, M., Gil-Guzman, E., Lopez, M. C., Sharma, R. K., Agarwal, A. and Larson, K. L. (2001) Characterization of subsets of human spermatozoa at different stages of maturation: implications in the diagnosis and treatment of male infertility. Hum. Reprod. 16, 1912–1921.
Gil-Guzman, E., Ollero, M., Lopez, M. C., Sharma, R. K., Alvarez, J. G. and Thomas, A. J. Jr. (2001) Differential production of reactive oxygen species by subsets of human spermatozoa at different stages of maturation. Hum. Reprod. 16, 1922–1930.
World Health Organization. Laboratory manual for the examination of human semen and sperm-cevical mucus interaction. 4th ed. New York. Cambridge University Press, 1999.
Kruger, T. F., Acosta, A. A., Simmons, K. F., Swanson, R. J., Matta, J. F. and Weeck, L. L. (1987) New method of evaluating sperm morphology with predictive value for human in vitro fertilization. Urology 30, 248–251.
Agarwal, A., Ikemoto, I. and Loughlin, K. R. (1992) Levels of reactive oxygen species before and after sperm preparation: comparison of swim-up and L4 filtration methods. Arch. Androl. 32, 169–174.
Agarwal, A., Ikemoto, I. and Loughlin, K. R. (1994) Effect of sperm washing on reactive oxygen species level in semen. Arch. Androl. 33, 157–162.
Shekarriz, M., Thomas, A. J. Jr. and Agarwal, A. (1995) A method of human semen centrifugation to minimize the iatrogenic sperm injuries caused by reactive oxygen species. Eur. Urol. 28, 31–35.
Lopes, S., Jurisicova, A., Sun, J. and Casper, R. F. (1998) Reactive oxygen species: a potential cause for DNA fragmentation in human spermatozoa. Hum. Reprod. 13, 896–900.
Zini, A., Finelli, A., Phang, D. and Jarvi, K. (2000) Influence of semen processing on human sperm DNA integrity. Urology 56, 1081–1084.
Aitken, R. J., Krausz, C. and Buckingham, D. (1994) Relationship between biochemical markers for residual sperm cytoplasm, reactive oxygen species generation and the presence of leukocytes and precursor germ cells in human sperm suspension. Mol. Reprod. Dev. 39, 268–279.
Rajasekaran, M., Hellstrom, W. J., Naz, R. K. and Sikka, S. C. (1995) Oxidative stress and interleukins in seminal plasma during leukocytospermia. Fertil. Steril. 64, 166–171.
Shekarriz, M., Sharma, R. K., Thomas, A. J. Jr. and Agarwal, A. (1995) Positive myeloperoxidase staining (Endtz Test) as an indicator of excessive reactive oxygen species formation in semen. J. Assist. Reprod. Genet. 12, 70–74.
Pasqualotto, F. F., Sharma, R. K., Agarwal, A., Nelson, D. R., Thomas, A. J. Jr, and Potts, J. M. (2000) Seminal oxidative stress in chronic prostatitis patients. Urology 55, 881–885.
Saran, M., Beck-Speier, I., Fellerhoff, B. and Bauer, G. (1999) Phagocytic killing of microorganisms by radical processes: consequences of the reaction of hydroxyl radicals with chloride yielding chlorine atoms. Free Radic. Biol. Med. 26, 482–490.
Ochsendorf, F. R. (1999) Infections in the male genital tract and reactive oxygen species. Hum. Reprod. 5, 399–420.
Sharma, R., Pasqualotto, F. F., Nelson, D. R., Thomas, A. J. Jr and Agarwal, A. (2001) Relationship between seminal white blood cell counts and oxidative stress in men treated at an infertility clinic. J. Androl. 22, 575–583.
Kovalski, N. N., de Lamirande, E. and Gagnon, C. (1992) Reactive oxygen species generated by human neutrophils inhibit sperm motility: protective effects of seminal plasma and scavengers. Fertil. Steril. 58, 809–816.
Saleh, R. A., Agarwal, A., Kandirali, E., Sharma, R. K., Thomas, A. J. Jr and Nada, E. A. (2002) Leukocytospermia is associated with increased reactive oxygen species production by human spermatozoa. Fertil. Steril. 78, 1215–1224.
Spitteler, G. (1993) Review: on the chemistry of oxidative stress. J. Lipid Mediat. 7, 77–82.
Jannsen, Y. M., Van-Houton, B., Borm, P. J. and Mossuran, B. T. (1993) Cell and tissue responses to oxidative damage. Lab. Invest. 69, 261–265.
de Lamirande, E., Jiang, H., Zini, A., Kodoma, H. and Gagnon, C. (1997). Reactive oxygen species (ROS) and sperm physiology. Rev. Reprod. 2, 48–54.
Sikka, S. C., Rajasekaran, M. and Hellstrom, W. J. G. (1995) Role of oxidative stress and antioxidants in male-infertility. J. Androl. 16, 464–468.
Halliwell, b. (1990) How to characterize a biological antioxidant. Free Radic. Res. Commun. 9, 1–32.
Aitken, R. J. and Fisher, H. (1994) Reactive oxygen species generation and human spermatozoa: the balance of benefit and risk. Bioassays 16, 259–267.
Lenzi, A., Cualosso, F., Gandini, L., Lombardo, F. and Dondero, F. (1993) Placebo controlled, double-blind, cross-over trial of glutathione therapy, in male infertility. Hum. Reprod. 9, 2044–2050.
Agarwal, A., Ikemoto, I. and Loughlin, K. R. (1994) Relationship of sperm parameters to levels of reactive oxygen species in semen specimens. J. Urol. 152, 107–110.
Ernster, L. Lipid peroxidation in biological membranes: mechanisms and implications. In: Active oxygen, lipid peroxides and antioxidants. Ed: Yagi K, CRC Press, Boca Raton, 1–38 (1993)
Darley-Usmar, V., Wiseman, H. and Halliwell, B. (1995) Nitric oxide and oxygen radicals: a question of balance. FEBS Letters 369, 131–135.
Taourel, D. B., Guerin, M. C. and Torreilles, J. (1992) Is melonaldehyde a valuable indicator of lipid peroxidation? Biochem. Pharmacol. 44, 985–988.
Armstrong, I. S., Rajasekaran, M., Chamulitrat, W., Gatti, P., Hellstrom, W. J. and Sikka, S. C. (1999) Characterization of reactive oxygen species induced effects on human spermatozoa movement and energy metabolism. Free Radic. Biol. Med. 26, 869–880.
de Lamirande, E. and Gagnon, C. (1995) Impact of reactive oxygen species on spermatozoa: a balancing act between beneficial and detrimental effects. Hum. Reprod. 10, 15–21.
Aitken, R. J. (1997) Molecular mechanisms regulating human sperm functions. Mol. Hum. Reprod. 3, 169–173.
Grivaeu, J. F., Dumont, E., Renard, B., Callegari, J. P. and Lannou, D. L. (1995) Reactive oxygen species, lipid peroxidation and enzymatic defense systems in human spermatozoa. J. Reprod. Fertil. 103, 17–26.
Twigg, J., Irvine, D. S. and Aitken, R. J. (1998) Oxidative damage to DNA in human spermatozoa does not, preclude pronucleous formation at intracytoplamic sperm injection. Hum. Reprod. 13, 1864–1871.
Duru, N. K., Morshedi, M. and Ochninger, S. (2000) Effects of hydrogen peroxide on DNA and plasma membrane integrity of human spermatozoa. Fertil. Steril. 74, 1200–1207.
Aitken, R. J. and Krausz, C. (2001) Oxidative stress, DNA damage and the Y chromosome. Reproduction 122, 497–506.
Aitken, R. J., West, K. M. and Buckingham, D. W. (1994) Leoukocyte infiltration into the human ejaculate and its association with semen quality, oxidative stress, and sperm function. J. Androl. 15, 343–352.
Fraga, G. G., Motchnik, P. A., Shigenaga, M. K., Helbrock, J. H., Jacob, R. A. and Ames (1991) Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. USA 88, 11003–11006.
Sun, J. G., Jurisicova, A. and Casoer, R. F. (1997) Detection of deoxyribonucleic acid fragmentation human sperm: correlation with fertilization in vitro. Biol. Reprod 56, 519–524.
Cummins, J. M., Jequier, A. M. and Raymons, K. (1994) Molecular biology of human male infertility: links with aging, mitochondrial genetics, and oxidative stress? Mol Rep and Dev 37, 345–362.
Aitken, R. J. and Baker, M. A. (2003) Oxidative stress and male reproductive biology. Reproduction, Fertility and development 16 (5), 581–588.
Sentman, C. L., Shutter, J. R., Hockenbery, D., Kanagawa, O. and Korsmeyer, S. J. (1991) Bcl-2 inhibits multiple forms of apoptosis but not negative selection in thyocytes. Cell 67, 879–888.
Maneesh, M., Jayalekshmi, H., Dutta, S., Chakrabarti, A. and Vasudevan, D. M. (2005) Role of oxidative stress in ethanol induced germ cell apoptosis—an experimental study in rats. Ind. J. of Clin. Biochem. 20 (2), 62–67.
Lee, J., Richburg, J. H., Yonkin, S. C. and Bockelheide, K. (1997) The Fas system is a key regulator of germ cell apoptosis in the testis. Endocrinology 138, 2081–2088.
Kane, D. J., Sarafian, T. A., Anton, R., Hahn, H., Gralla, E. B. and Valentine, J. S. (1993) Bcl-2 inhibition of neural death: decreased generation of reactive oxygen species. Science 262, 1274–1277.
Bar-Chama, N. and Lamb, D. (1994) Evaluation of sperm function. What is available in the modern andrology laboratory? Urologic Clinics of North Am. 21, 433–446.
Gagnon, C., Iwasaki, A., de Lamirande, E. and Kovalski, N. (1991) Reactive oxygen species and human spermatozoa. Ann NY Acad Sci 637, 436–444.
Rajasekaran, M., Hellstrom, W. J., Naz, R. K. and Sikka, S. C. (1995) Oxidative stress and interleukins in seminal plasma during leukocytospermia. Fertil. Steril. 64, 166–171.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maneesh, M., Jayalekshmi, H. Role of reactive oxygen species and antioxidants on pathophysiology of male reproduction. Indian J Clin Biochem 21, 80–89 (2006). https://doi.org/10.1007/BF02912918
Issue Date:
DOI: https://doi.org/10.1007/BF02912918