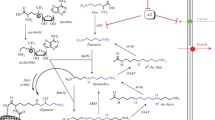
Abstract
The polyamines spermine, spermidine, and putrescine are small organic molecules one or more of which are present in all living organisms. Many natural products contain polyamine residues. Polyamines are synthesized by a highly regulated pathway from arginine or ornithine and also can be transported in and out of cells. Polyamines are degraded to a variety of compounds the functions of which are largely unknown. Polyamines influence the transcriptional and translational stages of protein synthesis, stabilize membranes, and, in mammalian systems, modulate neurophysiological functions and may act as intracellular messengers. However, at the molecular level the mode of action of the polyamines is largely unknown.
Similar content being viewed by others
References
Phillips, G. C. and Kuehn, G. D. (1991) Uncommon polyamines in plants and other organisms, inBiochemistry and Physiology of Polyamines in Plants, (Slocum, R. D. and Flores, H. E., eds.) CRC Press, Boca Raton FL, pp. 121–136.
Hamana, K. and Matsuzaki, S. (1992) Polyamines as a chemotaxonomic marker in bacterial systematics.Crit. Rev. Microbiol. 18, 261–283.
Hamana, K., Hamana, H., Niitsu, M., Samejima, K., Sakane, T., and Yokota, A. (1993) Tertiary and quaternary branched polyamines distributed in thermophilicSaccharococcus andBacillus.Microbios 75, 23–32.
Hamana, K., Hamana, H., Niitsu, M., Samejima, K., Sakane, T., and Yokota, A. (1994) Occurrence of tertiary and quaternary branched polyamines in thermophilic archaebacteria.Microbios 79, 109–119.
Hamana, K., Matsuzaki, S., Niitsu, M., and Samejima, K. (1994) Distribution of unusual polyamines in aquatic plants and gramineous seeds.Can. J. Bot. 72, 1114–1120.
Gaugas, J. M. (ed.) (1980)Polyamines in biomedical research. John Wiley, Chichester.
Morris, D. R. and Marton, L. J. (eds.) (1981)Polyamines in biology and medicine. Marcel Dekker, New York & Basel.
Tabor, H. and Tabor, C. W. (eds.) (1983)Polyamines. Methods Enzymol.84, Academic Press, New York and London.
Morgan, D. M. L. (1987) Polyamines.Essays Biochem. 23, 82–115.
Bachrach, U. and Heimer, Y. (eds.) (1989)Physiology of polyamines. CRC Press, Boca, Raton FL.
Pegg, A. E. (1986) Recent advances in the biochemistry of polyamines in eukaryotes.Biochem. J. 234, 249–262.
Pegg, A. E. (1988) Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy.Cancer Res. 48, 759–774.
Janne, J., Alhonen, L., and Leinonen, P. (1991) Polyamines: from molecular biology to clinical applications.Ann. Med. 23, 241–259.
Slocum, R. D. and Flores, H. E. (eds.) (1991)Biochemistry and physiology of polyamines in plants. CRC Press, Boca Raton FL.
Morgan, D. M. L. (1998) Polyamines: an introduction, inPolyamine Protocols (Morgan, D. M. L. ed.) Humana, Totowa, NJ. pp. 3–30.
Cohen, S. S. (1998)A Guide to the Polyamines Oxford University Press, New York.
van Leuwenhoek, A. (1678) Observationes D Anthonii Leuwenhoek de natis semine genitali animalculis.Phil. Trans. Roy. Soc. (London) 12, 1040–1043.
Seiler, N. (1980) Assay of polyamines in tissues and body fluids, inPolyamines in Biomedical Research (Gaugas, J. M., ed.) Wiley, Chichester, pp. 435–461.
Morgan, D. M. L. (ed.) (1998)Polyamine Protocols Humana, Totowa, NJ.
Liappis, N. (1972) Geschlechtsspezifische Unterschiede der Freien Aminosauren im Serum von Erdwachsenen.Z. Klin. Chem. Klin. Biochem. 10, 132–135.
Pegg, A. E. and McCann, P. P. (1982) Polyamine metabolism and function.Amer. J. Physiol. 243, C212-C221
Tabor, C. W. and Tabor, H. (1985) Polyamines in microorganisms.Microbiol. Rev. 49, 81–99.
Slocum, R. D., Kaur-Sawhney, R., and Galston, A. (1984) The physiology and biochemistry of polyamines in plants.Arch. Biochem. Biophys. 235, 283–303.
Smith, T. A. (1985) Polyamines.Ann. Rev. Plant Physiol. 36, 117–143.
Lortie, M. J., Novotny, W. F., Peterson, O. W., Vaallon, V., Malvey, K., Mendonca, M., Satriano, J., Insel, P., Thomson, S. C., and Blantz, R. C. (1996) Agmatine, a bioactive metabolite of arginine: production, degradation, and functional effects in the kidney of the rat.J. Clin. Invest. 97, 413–420.
Raasch, W., Regunathan, S., Li, G., and Reis, D. J. (1995) Agmatine, the bacterial amine, is widely distributed in mammalian tissues.Life Sci. 56, 2319–2330.
Tait, G. H. (1985) Bacterial polyamines, structures and biosynthesis.Biochem. Soc. Trans. 13, 316–318.
Canellakis, E. S., Viceps-Madore, D., Kyriakidis, D. A., and Heller, J. S. (1979) The regulation and function of ornithine decarboxylase and of the polyamines.Curr. Topics. Cell Regul. 15, 155–201.
Russell, D. H. (1983) Clinical relevance of polyamines.CRC Crit. Rev. Clin. Lab. Sci. 18, 261–311.
Hayashi, S. (ed.) (1989):Ornithine decarboxylase: biology, enzymology, and molecular genetics. Pergamon Press. Oxford and New York.
McCann, P. P. and Pegg, A. E. (1992) Ornithine decarboxylase as an enzyme target for therapy.Pharmacol. Ther. 54, 195–215.
Davis, R. H., Morris, D. R., and Coffino, P. (1992) Sequestered end products and enzyme regulation: the case of ornithine decarboxylase.Microbiol. Rev. 56, 280–290.
Applebaum, D. M., Dunlap, J. C., and Morris, D. R. (1977) Comparison of the biosynthetic and biodegradative ornithine decarboxylases ofEscherichia coli.Biochemistry 16, 1580–1584.
Poulin, R., Lu, L., Ackerman, B., Bey, P., and Pegg, A. E. (1992) Mechanisms of the irreversible inactivation of mouse ornithine decarboxylase by α-difluoromethylornithine.J. Biol. Chem. 267, 150–158.
Ghoda, L., van Daalen Wetters, T., Macrae, M., Ascherman, D., and Coffino, P. (1989) Prevention of rapid intracellular degradation of ODC by a carboxylterminal truncation.Science 243, 1493–1495.
Lu, L., Stanley, B. A., and Pegg, A. E. (1991) Identification of residues in ornithine decarboxylase essential for enzymic activity and for rapid protein turnover.Biochem. J. 277, 671–675.
Rogers, S., Wells, R., and Rechsteiner, M. (1986) Amino acid sequences common to rapidly degraded proteins: the PEST hypothesis.Science 234, 364–368.
Ghoda, L., Phillips, M. A., Bass, K. E., Wang, C. C., and Coffino, P. (1990) Trypanosome ornithine decarboxylase is stable because it lacks sequences found in the carboxy terminus of the mouse enzyme which target the latter for intracellular degradation.J. Biol. Chem. 265, 11823–11826.
Pegg, A. E., Seeley, J. E., Pösö, H., Della Ragione, F., and Zagon, I. S. (1982) Polyamine biosynthesis and interconversion in rodent tissues.Fed. Proc. 41, 3065–3072.
Tabor, C. W. and Tabor, H. (1984) Methionine adenosyltransferase (S-adenosylmethionine synthase) andS-adenosylmethionine decarboxylase.Adv. Enzymol. Relat. Areas Mol. Biol. 56, 251–282.
Pegg, A. E. and McCann, P. P. (1992)S-adenosylmethionine decarboxylase as an enzyme target for therapy.Pharmacol. Ther. 56, 359–377.
Slocum, R. D., Kaur Sawhney, R., and Galston, A. W. (1984) The physiology and biochemistry of polyamines in plants.Arch. Biochem. Biophys. 235, 283–303.
Bowman, W. H., Tabor, C. W., and Tabor, H. (1973) Spermidine biosynthesis. Purification and properties of propylamine transferase fromEscherichia coli.J. Biol. Chem. 248, 2480–2486.
Hirasawa, E. and Suzuki, Y. (1983) Biosynthesis of spermidine in maize seedlings.Phytochemistry 22, 103–106.
Samejima, K. and Yamanoha, B. (1982) Purification of spermidine synthase from rat ventral prostate by affinity chromatography on immobilizedS-adenosyl(5′)-3-thiopropylamine.Arch. Biochem. Biophys. 216, 213–222.
Pajula, R. L., Raina, A., and Eloranta, T. (1979) Polyamine synthesis in mammalian tissues: isolation and characterisation of spermine synthase from bovine brain.Eur. J. Biochem. 101, 619–626.
Yamanoha, B., Samejima, K., Nakajima, T., and Yasuhara, T. (1984) Differences between homogenous spermidine synthases isolated from rat and pig liver.J. Biochem. (Tokyo). 96, 1273–1281.
Raina, A., Hyvonen, T., Eloranta, T., Voutilainen, M., Samejima, K., and Yamanoha, B. (1984) Polyamine synthesis in mammalian tissues. Isolation and characterization of spermidine synthase from bovine brain.Biochem. J. 219, 991–1000.
Kajander, E. O., Kauppinen, L. I., Pajula, R. L., Karkola, K., and Eloranta, T. O. (1989) Purification and partial characterization of human polyamine synthases.Biochem. J. 259, 879–886.
Wahlfors, J., Alhonen, L., Kauppinen, L., Hyvonen, T., Janne, J., and Eloranta, T. O. (1990) Human spermidine synthase: cloning and primary structure.DNA Cell Biol. 9, 103–103.
Casero, R. A., Jr. and Pegg, A. E. (1993) Spermidine/spermineN 1-acetyltransferase—the turning point in polyamine metabolism.FASEB J. 7, 653–661.
Haywood, G. W. and Large, P. J. (1985) The occurrence, subcellular localization and partial purification of diamine acetyltransferase in the yeast Candida boidinii grown on spermidine or putrescine as sole nitrogen source.Eur. J. Biochem. 148, 277–283.
Della Ragione, F. and Pegg, A. E. (1983) Studies of the specificity and kinetics of rat liver spermidine/spermineN 1-acetyltransferase.Biochem. J. 213, 701–706.
Hiramatsu, K., Sugimoto, M., Kamei, S., Hoshino, M., Kinoshita, K., Iwasaki, K., and Kawakita, M. (1997) Diagnostic and prognostic usefulness ofN 1,N 8-diacetylspermidine andN 1,N 12-diacetylspermine in urine as novel markers of malignancy.J. Cancer Res. Clin. Oncol. 123, 539–545.
Hiramatsu, K., Sugimoto, M., Kamei, S., Hoshino, M., Kinoshita, K., Iwasaki, K., and Kawakita, M. (1995) Determination of amounts of polyamines excreted in urine: demonstration ofN 1,N 8-diacetylspermidine andN 1,N 12-diacetylspermine as components commonly occurring in normal human urine.J. Biochem. (Tokyo) 117, 107–112.
Della Ragione, F. and Pegg, A. E. (1982) Purification and characterization of spermidine/spermineN 1-acetyltransferase from rat liver.Biochemistry 21, 6152–6158.
Libby, P. R., Ganis, B., Bergeron, R. J., and Porter, C. W. (1991) Characterization of human spermidine/spermineN 1-acetyltransferase purified from cultured melanoma cells.Arch. Biochem. Biophys. 284, 238–244.
Van den Berg, G. A., Muskiet, F. A. J., Kingma, A. W., Van Der Slik, W., and Halie, M. R. (1986) Simultaneous gas-chromatographic determination of free and acetyl-conjugated polyamines in urine.Clin. Chem. 32, 1930–1930.
Van den Berg, G. A., Kingma, A. W., Visser, G. H., and Muskiet, F. A. (1988) Gestational-age-dependent concentrations of polyamines, their conjugates and metabolites in urine and amniotic fluid.Br. J. Obstet. Gynaecol. 95, 669–675.
Casero, R. A. Jr., Celano, P., Ervin, S. J., Wiest, L., and Pegg, A. E. (1990) High specific induction of spermidine/spermine N1-acetyltransferase in a human large cell lung carcinoma.Biochem. J. 270, 615–620.
Xiao, L., Celano, P., Mank, A. R., Pegg, A. E., and Casero, R. A. Jr. (1991) Characterization of a full-length cDNA which codes for the human spermidine/spermineN 1-acetyltransferase.Biochem. Biophys. Res. Commun. 179, 407–415.
Pegg, A. E., Stanley, B. A., Wiest, L., and Casero, R. A., Jr. (1992) Nucleotide sequence of hamster spermidine/spermine-N 1-acetyltransferase cDNA.Biochim. Biophys. Acta 1171, 106–108.
Fogel-Petrovic, M., Kramer, D. L., Ganis, B., Casero, R. A., Jr., and Porter, C. W. (1993) Cloning and sequence analysis of the gene and cDNA encoding mouse spermidine/spermineN 1-acetyltransferase—A gene uniquely regulated by polyamines and their analogs.Biochim. Biophys. Acta 1216, 255–264.
Libby, P. R. (1978) Calf liver nuclearN-acetyltransferases.J. Biol. Chem. 253, 233–237.
Libby, P. R. (1980) Rat liver nuclearN-acetyltransferases: separation of two enzymes with both histone and spermidine acetyltransferase activity.Arch. Biochem. Biophys. 203, 384–389.
Erwin, B. G., Persson, L., and Pegg, A. E. (1984) Differential inhibition of histone and polyamine acetylases by multisubstrate analogues.Biochemistry,23, 4250–4255.
Desiderio, M. A., Mattei, S., Biondi, G., and Colombo, M. P. (1993) Cytosolic and nuclear spermidine acetyltransferases in growing NIH 3T3 fibroblasts stimulated with serum or polyamines: relationship to polyamine-biosynthetic decarboxylases and histone acetyltransferase.Biochem. J. 293, 475–479.
Libby, P. R. (1978) Properties of an acetylspermidine deacetylase from rat liver.Arch. Biochem. Biophys. 188, 360–363.
Blankenship, J. (1978) Deacetylation ofN 8-acetylspermidine by subcellular fractions of rat tissue.Arch. Biochem. Biophys. 189, 20–27.
Marchant, P., Manneh, V. A., and Blankenship, J. (1986)N 1-acetylspermidine is not a substrate forN-acetylspermidine deacetylase.Biochim. Biophys. Acta 881, 297–299.
Morgan, D. M. L. (1980) Polyamine oxidases, inPolyamines in Biomedical Research, (Gaugas, J. M. ed.) Wiley, Chichester. pp. 285–302.
Morgan, D. M. L. (1989) Polyamine oxidase and oxidised polyamines, inPhysiology of the Polyamines vol I (Bachrach, U. and Heimer, Y., eds.) CRC Press, Florida, Boca Raton FL, pp. 203–229.
Mondovi, B. (ed.) (1985)Structure and Functions of Amine Oxidases. CRC Press, Boca Raton.
Nomenclature Committee. (1992)Enzyme Nomenclature 1992. Academic Press, San Diego.
Carroll, L. (1872) Through the looking glass and what Alice found there, inThe Complete Works of Lewis Carroll Nonsuch Press, London (1939) pp. 196
Morgan, D. M. L. (1998) Polyamine oxidases-enzymes of unknown function?Biochem. Soc. Trans. 26, 586–590.
Federico, R. and Angelini, R. (1991) Polyamine catabolism in plants, inBiochemistry and Physiology of Polyamines in Plants (Slocum, R. D. and Flores, H. E., eds.) CRC Press, Boca Raton, FL, pp. 41–56.
Gahl, W. A., Vale, A. M. and Pitot, H. C. (1982) Spermidine oxidase in human pregnancy serum. Probable identity with diamine oxidase.Biochem. J. 201, 161–164.
Morgan, D. M. L. (1985) Human pregnancy-associated polyamine oxidase: partial purification and properties.Biochem. Soc. Trans. 13, 351–352.
Seiler, N., Bolkenius, F. N., and Knodgen, B. (1985) The influence of catabolic reactions on polyamine excretion.Biochem. J. 225, 219–226.
Klinman, J. P. and Mu, D. (1994) Quinoenzymes in biology.Annu. Rev. Biochem. 63, 299–344.
Tabor, C. W., Tabor, H., and Bachrach, U. (1964) Identification of the aminoaldehydes produced by the oxidation of spermine and spermidine with purified plasma amine oxidase.J. Biol. Chem. 239, 2194–2203.
Tabor, C. W., Tabor, H., and Rosenthal, S. M. (1954) Purification of amine oxidase from beef plasma.J. Biol. Chem. 208, 645–661.
Israel, M., Rosenfield, J. S., and Modest, E. J. (1964): Analogs of spermine and spermidine. J. Synthesis of polymethylene-polyamines by reduction and cyanoethylated α,ω-alkylenediamines.J. Med. Chem. 7, 710–716.
Gahl, W. A. and Pitot, H. C. (1982) Polyamine degradation in foetal and adult bovine serum.Biochem. J. 202, 603–611.
Isobe, K., Tani, Y., Yamada, H., and Hiromi, K. (1980) Determination of polyamines with immobilised beef plasma amine oxidase.Agric. Biol. Chem. 44, 615–619.
Mamont, P. S., Seiler, N., Siat, M., Joder-Ohlenbusch, A.-M., and Knodgen, B. (1981) Metabolism of acetyl derivatives of polyamines in cultured polyamine-deficient rat hepatoma cells.Med. Biol. 59, 347–347.
Castellano, F. N., He, Z. and Greenaway, F. T. (1993) Hydroxyl radical production in the reactions of copper-containing amine oxidases with substrates.Biochim. Biophys. Acta 1157, 162–166.
Alarcon, R. A. (1964) Isolation of acrolein from incubated mixtures of spermine with calf serum and its effect on mammalian cells.Arch. Biochem. Biophys. 106, 240–242.
Alarcon, R. A. (1970) Acrolein. IV. Evidence for the formation of the cytotoxic aldehyde acrolein from enzymatically oxidised spermine or spermidine.Arch. Biochem. Biophys. 137, 365–372.
Kimes, B. W. and Morris, D. R. (1971) Inhibition of nucleic acid and protein synthesis inEscherichia coli by oxidised polyamines and acrolein.Biochem. Biophys. Acta. 228, 235–244.
Huen, G., Bock, K., and Jensen, A. L., (1994) HPLC and NMR investigation of the serum amine oxidase catalyzed oxidation of polyamines.Acta Chem. Scand. 48, 52–60. 1994.
Fernandez, C., Sharrard, R. M., Talbot, M., Reed, B. D., and Monks, N. (1995) Evaluation of the significance of polyamines and their oxidases in the aetiology of human cervical carcinoma.Br. J. Cancer 72, 1194–1199.
Averill-Bates, D. A., Agostinelli, E., Przybytkowski, E., Mateescu, M. A., and Mondovi, B. (1993) Cytotoxicity and kinetic analysis of purified bovine serum amine oxidase in the presence of spermine in Chinese hamster ovary cells.Arch. Biochem. Biophys. 300, 75–79.
Israel, M., Zoll, E. C., Muhammad, N., and Modest, E. J. (1973) Synthesis and antitumor evaluation of the presumed cytotoxic metabolites of spermine andN, N′-bis(3-aminopropyl)nonane-1,9-diamine.J. Med. Chem. 16, 1–5.
Hölttä, E. (1977) Oxidation of spermidine and spermine in rat liver: purification and properties of polyamine oxidase.Biochemistry 16, 91–100.
Seiler, N., Bolkenius, F. N., Knodgen, B., and Mamont, P. (1980) Polyamine oxidase in rat tissues.Biochem. Biophys. Acta 615, 480–488.
Rinaldi, A., Floris, G., and Giartosio, A. (1985): Plant amine oxidases, inStructure and Functions of Amine Oxidases (Mondovi B., ed.) CRC Press, Boca Raton, pp. 51–51.
Tavladoraki, P, Schinina, M. E., Cecconi, F., DiAgostino, S., Rea, G., Mariottini, P., Federico, R., and Angelini, R. (1998) Maize polyamine oxidase: primary structure from protein and cDNA sequencing.FEBS Lett. 426, 62–66.
Green, J., Haywood, G. W., and Large, P. J. (1983) Serological differences between the multiple amine oxidases of yeasts and comparison of the specificities of the purified enzymes fromCandida utilis andPichia pastoris.Biochem. J. 211, 481–493.
Kumagai, H. and Yamada, H. (1985) Bacterial and fungal amine oxidases, inStructure and Functions of Amine Oxidases (Mondovi, B., ed.) CRC Press, Boca Raton. pp. 37–37.
Large, P. J. (1992) Enzymes and pathways of polyamine breakdown in microorganisms.FEMS Microbiol. Rev. 88, 249–262.
Mezl, V. A., Fournier, L. A., and Garber, P. M. (1986)N 1-monoacetylation abolishes the inhibitory effect of spermine and spermidine in the reticulocyte lysate translation system.Int. J. Biochem. 18, 705–711.
Seiler, N. (1987) Functions of polyamine acetylation.Can. J. Physiol. Pharmacol. 65, 2024–2035.
Seiler, N., Knodgen, B., and Haegele, K. (1982)N-(3-Aminopropyl)pyrrolidon-2-one, a product of spermidine catabolism in vivo.Biochem. J. 208, 189–197.
Muskiet, F. A. J., Stratingh, C. M., and Fremouw-Ottevangers, D. C. (1982) Mass spectrometric identification of isoputreanine, a metabolite of spermidine and/or spermine, in human urine.J. Chromatogr. 230, 142–147.
Muskiet, F. A. J., Dorhout, B., van den Berg, G. A., and Hessels, J. (1995) Investigation of polyamine metabolism by high-performance liquid chromatographic and gas chromatographic profiling methods.J. Chromatogr. 667, 189–198.
Van den Berg, G. A., Elzinga, H., Nagel, G. T., Kingma, A. W., and Muskiet, F. A. J. (1984) Catabolism of polyamines in the rat. Polyamines and their non-alpha-amino acid metabolites.Biochim. Biophys. Acta 802, 175–187.
Kawase, H., Hasegawa, T., Hashimoto, H., Noto, T., and Nakajima, T. (1994) A study on the metabolism of spermidine in mammals: Purification and identification of a newly identified metabolite, 2-oxo-1-pyrrolidinepropionic acid, in rat urine.J. Biochem. (Tokyo) 115, 356–361.
Seiler, N., Bolkenius, F. N., and Rennert, O. M. (1981) Interconversion, catabolism and elimination of the polyamines.Med. Biol. 59, 334–346.
Seiler, N. (1992) Polyamine catabolism and elimination by the vertebrate organism, inPolyamines in the gastrointestinal tract (Dowling, R. H., Folsch, U. R., and Loser, C., eds.) Kluwer, Dordrecht, pp. 65–85.
Mamont, P. S., Bohlen, P., McCann, P. P., Bey, P., Schuber, F., and Tardif, C. (1976) α-Methylornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatome cells in culture.Proc. Natl. Acad. Sci. USA 73, 1626–1630.
Morgan, D. M. L. (1990) Polyamines and cellular regulation: perspectives.Biochem. Soc. Trans. 18, 1080–1084.
Russell, D. H. (1985) Ornithine decarboxylase: a key regulatory enzyme in normal and neoplastic growth.Drug Metab. Rev. 16, 1–88.
Law, G. L., Li, R.-S., and Morris, D. R. (1995) Transcriptional control of the ODC gene, inPolyamines: regulation and molecular interaction (Casero, R. A., Jr., ed.), R. G. Landes, Austin, TX, pp. 5–26.
Hayashi, S. (1995) Antizyme-dependent degradation of ornithine decarboxylase.Essays Biochem. 30, 37–47.
Stanley, B. A. (1995) Mamalian S-adenosylmethionine decarboxylase, regulation and processing, inPolyamines: regulation and molecular interaction (Casero, R. A., Jr., ed.) R. G. Landes, Austin, TX, pp. 27–75.
Persson, L., Khomutov, A. R. and Khomutov, R. M. (1989) Feedback regulation of S-adenosylmethionine decarboxylase synthesis.Biochem. J. 257, 929–931.
White, M.W., Degnin, C., Hill, J. and Morris, D. R. (1990) Specific regulation by endogenous polyamines of translational initiation ofS-adenosylmethionine decarboxylase mRNA in Swiss 3T3 fibroblasts.Biochem. J. 268, 657–660.
Xiao, L. and Casero, R. A. Jr. (1995) Regulation of spermidine/spermine N1-acetyltransferase, inPolyamines: regulation and molecular interaction (Casero, R. A., Jr. ed.) R. G. Landes, Austin, TX, pp. 77–99.
Seiler, N. and Dezeure, F. (1990) Polyamine transport in mammalian cells.Int. J. Biochem. 22, 211–211.
Khan, N. A., Quemener, V., and Moulinoux, J.-P. (1991) Polyamine membrane transport regulation.Cell Biol. Int. Rep. 15, 9–24.
Seiler, N., Delcros, J.-G. and Moulinoux, J.-P. (1996) Polyamine transport in mammalian-cells—an update.Int. J. Biochem. Cell Biol. 28, 843–861.
Morgan, D. M. L. (1992) Uptake of polyamines by human endothelial cells. Characterization and lack of effect of agonists of endothelial function.Biochem. J. 286, 413–417.
Bogle, R. G., Mann, G. E., Pearson, J. D., and Morgan, D. M. L. (1994) Endothelial polyamine uptake: selective stimulation by L-arginine deprivation or polyamine depletion.Am. J. Physiol. Cell Physiol. 266, C776-C783.
Hayashi, S., Murakami, Y. and Matsufuji, S. (1996) Ornithine decarboxylase antizyme: a novel type of regulatory protein.Trends. Biochem. Sci. 21, 27–30. 1996.
Coffino, P. and Poznanski, A. (1991) Killer polyamines?J. Cell. Biochem. 45, 54–58.
Brunton, V. G., Grant, M. H., and Wallace, H. M. (1991) Mechanisms of spermine toxicity in baby hamster kidney (BHK) cells. The role of amine oxidases and oxidative stress.Biochem. J. 280, 193–198.
Casero, R. J., Jr. (ed.) (1995)Polyamines: regulation and molecular interaction. R. G. Landes. Austin, TX.
Nishioka, K. (ed.) (1996)Polyamines in cancer: basic mechanisms and clinical approaches. R. G. Landes, Austin, TX.
Shipe, J. R., Savory, J., and Wills, M. R. (1981) Polyamines as tumor markers.CRC Crit. Rev. Clin. Lab. Sci. 5, 91–95.
Scalabrino, G. and Ferioli, M. E. (1982) Polyamines in mammalian tumors. Part I.Adv. Cancer Res. 35, 151–268.
Scalabrino, G. and Ferioli, M. E. (1982) Polyamines in mammalian tumors. Part II.Adv. Cancer Res. 36, 1–102.
Oredsson, S. M. and Marton, L. J. (1982) Polyamines: the elusive cancer markers.Clinics. Lab. Med. 2, 507–518.
Scalabrino, G., Lorenzini, E. C., and Ferioli, M. E. (1991) Polyamines and mammalian hormones. Part I: biosynthesis, interconversion and hormone effects.Mol. Cell. Endocrinol. 77, 1–35.
Scalabrino, G. and Lorenzini, E. C. Polyamines and mammalian hormones. (1991) Part II paracrine signals and intracellular regulators.Mol. Cell. Endocrinol. 77, 37–56.
Pegg, A. E. (1988) Polyamine metabolism and its importance in neoplastic growth and as a target for chemotherapy.Cancer Res. 48, 759–759.
Marton, L. J. and Pegg, A. E. (1995) Polyamines as targets for therapeutic intervention.Annu. Rev. Pharmacol. Toxicol. 35, 55–91.
Nishioka, K., Mitchell, M. F., and Ajani, J. A. (1996): Clinical studies of polyamines and their metabolites, inPolyamines in cancer: basic mechanisms and clinical approaches (Nishioka, K., ed.) R. G. Landes, Austin TX, pp. 251–278.
McCann, P. P., Pegg, A. E., and Sjoerdsma, A. (1987)Inhibition of polyamine metabolism. Academic Press, San Diego.
Seiler, N. (1991) Pharmacological properties of the natural polyamines and their depletion by biosynthesis inhibitors as a therapeutic approach.Prog. Drug Res. 37, 107–159. 1991.
Regenass, U., Mett, H., Stanek, J., Mueller, M., Kramer, D., and Porter, C. W. (1994) CGP 48664, a newS-adenosylmethionine decarboxylase inhibitor with broad spectrum antiproliferative and antitumor activity.Cancer Res. 54, 3210–3217.
Kramer, D. L. (1996) Polyamine inhibitors and analogs, inPolyamines in cancer: basic mechanisms and clinical approaches (Nishioka, K., ed.) R. G. Landes, Austin TX, pp. 151–189.
Fairlamb, A. H. (1990) Trypanothione metabolism and rational approaches to drug design.Biochem. Soc. Trans. 18, 717–717.
Fairlamb, A. H. (1990) Future prospects for the chemotherapy of human trypanosomiasis. 1. Novel approaches to the chemotherapy of trypanosomiasis.Trans. R. Soc. Trop. Med. Hyg. 84, 613–617.
Bergeron, R. J. (1986) Methods for the selective modification of spermidine and its homologues.Accounts Chem. Res. 19, 105–105.
Marton, L. J. and Morris, D. R. (1998) Molecular and cellular functions of the polyamines, inInhibition of Polyamine Metabolism (McCann, P. P., Pegg, A. E., and Sjoerdsma, A., eds.) Academic Press, San Diego, pp. 79–105.
Sarhan, S. and Seiler, N. (1989) On the subcellular localization of the polyamines.Biol. Chem. Hoppe-Seyler 370, 1279–1284.
Lounasmaa, M. (1988) The tropane alkaloids.The Alkaloids 33, 1–81.
Leete, E. (1990) Recent developments in the biosynthesis of the tropane alkaloids.Planta Med. 56, 339–352.
Niwa, H., Watanabe, M., and Yamada, K. (1993) Monodontamides A, B, and C, three new putrescine alkaloids from the marine gastropod molluscMonodonta labio (Linné).Tetrahedron Lett. 34, 7441–7444.
Goggisberg, A. and Hesse, M. (1983) Putrescine, spermidine, spermine, and related polyamine alkaloids.The Alkaloids 22, 85–188.
Saccomano, N. A., Volkmann, R. A., Jackson, H., and Parks, T. N. (1989) Polyamine spider toxins: unique pharmacological tools.Ann. Rep. Med. Chem. 24, 287–293.
Jasys, V. J., Kelbaugh, P. R., Nason, D. M., Phillips, D., Rosnack, K. J., Saccoman, N. A., Stroh, J. G., and Volkmann, R. A. (1990) Isolation, structure elucidation, and synthesis of novel hydroxylamine-containing polyamines from the venom of the Agelenopsis aperta spider.J. Am. Chem. Soc. 112, 6696–6704.
Fairlamb, A. H. and Cerami, A. (1992) Metabolism and functions of trypanothione in the kinetoplastida.Annu. Rev. Microbiol. 46, 695–729.
Hohenschutz, L. D., Bell, E. A., Jewess, P. J., Leworthy, D. P., Pryce, R. J., Arnold, E., and Clardy, J. (1981) Castanospermine, a 1,6,7,8-tetrahydroxyoctahydro-indolizine alkaloid, from seeds ofCastanospermum australe.Phytochemistry 20, 811–814.
Saxton, J. E. (1983) The Aspidosperma group, inHeterocyclic Compounds, Vol. 25 pt. 4. The monoterpenoid Indole Alkaloids (Saxton, J. E., ed.) John Wiley, New York, pp. 331–437.
Cordell, G. A. (1983) The bisindole alkaloids, inHeterocyclic Compounds, Vol. 25 pt. 4. The monoterpenoid Indole Alkaloids (Saxton, J. E., ed.) John Wiley, New York, pp. 539–728.
Schuber, F. (1989) Influence of polyamines on membrane functions.Biochem. J. 260, 1–10.
Morgan, D. M. L., Coade, S. B., and Pearson, J. D. (1990) Polyamines stimulate calcium uptake by human vascular endothelial cells.Biochem. Soc. Trans. 18, 1223–1224.
Groblewski, G. E., Hargittai, P. T., and Seidel, E. R. (1992) Ca2+/calmodulin regulation of putrescine uptake in cultured gastrointestinal epithelial cells.Am. J. Physiol. 262, C1356-C1363.
Khan, N. A., Sezan, A., Quemener, V., and Moulinoux, J.-P. (1993) Polyamine transport regulation by calcium and calmodulin: role of Ca2+-ATPase.J. Cell. Physiol. 157, 493–501.
Williams, K., Romano, C., Dichter, M. A., and Molinoff, P. B. (1991) Modulation of the NMDA receptor by polyamines.Life Sci. 48, 469–498.
Williams, K. (1994) Modulation of theN-methyl-D-aspartate receptor by polyamines: molecular pharmacology and mechanisms of action.Biochem. Soc. Trans. 22, 884–887.
Carter, C. (ed.) (1994)Neuropharmacology of polyamines. Academic Press, San Diego.
Williams, K. (1997) Interactions of polyamines with ion channels.Biochem. J. 325, 289–297.
Park, M. H., Wolff, E. C., and Folk, J. E. (1993) Hypusine: its post-translational formation in eukaryotic initiation factor 5A and its potential role in cellular regulation.BioFactors 4, 95–104.
Morgan, D. M. L. (1994) Polyamines, arginine and nitric oxide.Biochem. Soc. Trans. 22, 879–883.
Baydoun, A. R. and Morgan, D. M. L. (1998) Inhibition of ornithine decarboxylase potentiates nitric oxide production in LPS-activated J774 cells.Br. J. Pharmacol. 125, 1511–1516.
Moncada, S., Palmer, R. M. J., and Higgs, E. A. (1991) Nitric oxide: physiology, pathophysiology and pharmacology.Pharmacol. Rev. 43, 109–142.
Yin, Z. L. and Dusting, G. J. (1997) A nitric oxide donor (spermine-nonoate) prevents the formation of neointima in rabbit carotid artery.Clin. Exp. Pharmacol. Physiol. 24, 436–438.
Tabor, H., Tabor, C. W., and DeMeis, L. (1971) Chemical synthesis ofN-acetyl-1,4-diaminobutane,N 1-acetylspermidine, andN 8-acetylspermidine.Methods Enzymol. 17B, 829–833.
Smith, T. A. and Barker, J. H. (1988) The di- and polyamine oxidases of plants.Adv. Exp. Med. Biol. 250, 573–587.
Yamada, H., Adachi, O., and Ogata, K. (1965) Putrescine oxidase, a diamine oxidase requiring flavin adenine dinucleotide.Agr. Biol. Chem. 29, 1148–1149.
Ishizuka, H., Horinouchi, S., and Beppu, T. (1993) Putrescine oxidase ofMicrococcus rubens: Primary structure andEscherichia coli.J. Gen. Microbiol. 139, 425–432.
Yamada, H., Adachi, O., and Ogata, K. (1965) Amine oxidases of microorganisms: part IV. Further properties of amine oxidase ofAspergillus niger.Agr. Biol. Chem. 29, 912–917.
Vianello, F., Di Paolo, M. L., Stevanato, R., Gasparini, R., and Rigo, A. (1993) Purification and characterization of amine oxidase from soybean seedlings.Arch. Biochem. Biophys.,307, 35–39.
Suzuki, Y. and Yanagisawa, H. (1980) Purification and properties of maize polyamine oxidase—a flavoprotein.Plant Cell Physiol. 21, 1085–1094.
Suzuki, Y. and Hagiwara, M. (1993) Purification and characterization of diamine oxidase fromZea mays shoots.Phytochemistry 33, 995–998.
Federico, R., Alisi, C., Forlani, F., and Angelini, R. (1989) Purification and characterization of oat polyamine oxidase.Phytochemistry 28, 2045–2046.
Tipping, A. J. and McPherson, M. J. (1995) Cloning and molecular analysis of the pea seedling copper amine oxidase.J. Biol. Chem. 270, 16,939–16,946.
Müller, S. and Walter, R. D. (1992) Purification and characterization of polyamine oxidase fromAscaris suum.Biochem. J. 283, 75–80.
Pettersson, G. (1985) Plasma amine oxidase, inStructure and Functions of Amine Oxidases (Mondovi, B. ed.) CRC Press, Boca Raton, FL, pp. 105–120.
Gasparayan, V. K. and Nalbandyan, R. M. (1990) Purification and fluorescent properties of flavin-containing liver polyamine oxidase.Biochemistry USSR,55, 1223–1227.
Crabbe, M. J. C., Waight, R. D., Bardsley, W. G., Barker, R. W., Kelley, I., and Knowles, P. F. (1976) Human placental diamine oxidase. Improved purification and characterization of a copper- and manganese-containing amine oxidase with novel substrate specificity.Biochem J. 155, 679–687.
McIntyre, W. S. and Hartmann, C. (1993) Copper-containing amine oxidases, inPrinciples and Applications of Quinoproteins (Davidson, V. L. ed.) Marcel Dekker, New York, pp. 97–171.
Lee, Y. and Sayre, L. M. (1998) Reaffirmation that metabolism of polyamines by bovine plasma amine oxidase occurs strictly at the amino termini.J. Biol. Chem. 273, 19,490–19,494.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morgan, D.M.L. Polyamines. Mol Biotechnol 11, 229–250 (1999). https://doi.org/10.1007/BF02788682
Issue Date:
DOI: https://doi.org/10.1007/BF02788682