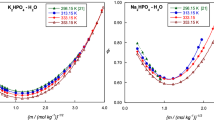
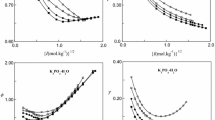
Abstract
Published thermodynamic data measured in aqueous mixtures of sodium or potassium dihydrogen phosphate with hydrogen phosphate and chloride at 25°C were used to test recently developed methods for calculation of the pH of phosphate buffer solutions. Equations for ionic activity coefficients are used in these methods. It is shown that all data used in the tests up to an ionic strength of about 0.5 mol-kg-1 can be accurately predicted by the two methods recommended. In one of these methods, equations of the Hückel type are used for ionic activity coefficients and in the other equations of the Pitzer type. Several sets of phosphate buffer solutions are recommended,e.g., for calibrations of glass electrode cells. In the recommended sets, the pH of the buffer solutions can be calculated either by the Hückel or Pitzer method, and the pH predictions of these methods agree in most cases within 0.005 at least up to ionic strengths of about 0.2 mol-kg-1. The pH values of the two primary pH standards endorsed by IUPAC based on aqueous mixtures of KH2PO4 and Na2HPO4,i.e., pH values of 6.865 and 7.413, can also be accurately predicted by the equations recommended in this study.
Similar content being viewed by others
References
E. J. Cohn,J. Am. Chem. Soc. 49, 173 (1927).
E. A. Guggenheim and T. D. Schindler,J. Phys. Chem. 38, 533 (1934).
D. I. Hitchcock and A. C. Taylor,J. Am. Chem. Soc. 59, 1812 (1937).
R. G. Bates and S. F. Acree,J. Res. Natl. Bur. Stand. 30, 129 (1943).
R. G. Bates and S. F. Acree,J. Res. Natl. Bur. Stand. 34, 373 (1945).
R. G. Bates,J. Res. Natl. Bur. Stand., Sect. A 66, 179 (1962).
R. G. Bates and E. A. Guggenheim,Pure Appl. Chem. 1, 163 (1960).
A. K. Covington, R. G. Bates, and R. A. Durst,Pure Appl. Chem. 57, 531 (1985).
M. J. G. Lito, M. F. G. CamŌes, M. I. A. Ferra, and A. K. Covington,Anal. Chim. Acta 239, 129 (1990).
A. K. Covington and M. I. A. Ferra,J. Solution Chem. 23, 1 (1994).
C. Y. Chan, Y. W. Eng, and K. S. Eu,J. Chem. Eng. Data 40, 685 (1995).
J. I. Partanen, M. H. KÄrki, and P. M. Juusola,Acta Chem. Scand. 49, 865 (1995).
J. I. Partanen, P. M. Juusola, and P. O. Minkkinen,Acta Chem. Scand. 49, 163 (1995).
J. I. Partanen, P. M. Juusola, and P. O. Minkkinen,Acta Polytech. Scand. Chem. Technol. Ser. 231, 1 (1995).
K. S. Pitzer and L. F. Silvester,J. Solution Chem. 5, 269 (1976).
D. G. Archer and P. Wang,J. Phys. Chem. Ref. Data 19, 371 (1990).
K. S. Pitzer and G. Mayorga,J. Phys. Chem. 77, 2300 (1973).
K. S. Pitzer and J. J. Kim,J. Am. Chem. Soc. 96, 5701 (1974).
J. I. Partanen and P. O. Minkkinen,Acta Chem. Scand. 50, 1081 (1996).
R. G. Bates,J. Res. Natl. Bur. Stand. 47, 127 (1951).
L. G. Sillén and A. E. Martell,Stability Constants of Metal-Ion Complexes, Special Pub. No. 17 (The Chemical Society, London, 1964) p. 180.
R. G. Bates and J. B. Macaskill,Pure Appl. Chem. 50, 1701 (1978).
H. S. Harned and R. W. Ehlers,J. Am. Chem. Soc. 54, 1350 (1932).
V. E. Bower, M. Paabo, and R. G. Bates,J. Res. Natl. Bur. Stand. Sect. A 65, 267 (1961).
R. G. Bates, C. A. Vega, and D. R. White,Anal. Chem. 50, 1295 (1978).
C. A. Vega, M. Romero, and R. G. Bates,J. Chem. Eng. Data 39, 294 (1994).
R. G. Bates and V. E. Bower,J. Res. Natl. Bur. Stand. 53, 283 (1954).
O. Johansson and M. Wedborg,Mar. Chem. 8, 57 (1979).
J. P. Hershey, M. Fernandez, and F. J. Millero,J. Solution Chem. 18, 875 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Partanen, J.I., Minkkinen, P.O. Equations for calculation of the pH of buffer solutions containing sodium or potassium dihydrogen phosphate, sodium hydrogen phosphate, and sodium chloride at 25°C. J Solution Chem 26, 709–727 (1997). https://doi.org/10.1007/BF02767623
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02767623