Abstract
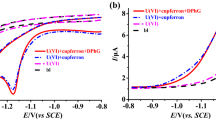
The glass/Ag0, Ag2S electrode applicable for in situ determination of H2S was tested in solutions of inert electrolytes. No effects on sensitivity, selectivity and slope were found up to an ionic strength of ≈0.7 (artificial seawater). In salty brines such as Dead Sea water or NaClsat. solution a positive potential shift and a slope increase was observed. This corresponds, presumably, to salting-out of the gas. Within the observed precision range of the detection method (+-3%) the electrode allows the measurement of H2Sconcentrations (lower limit of detection: 10−5.5 M Stot). Nevertheless, as a consequence of its half cell combination, the electrode could be a means for the direct detection of the salting coefficient of H2S in hypersaline media (I≫0.1).
Similar content being viewed by others
References
Battino, R., and Clever, H. L.: The solubility of gases in liquids, Chem. Rev.66, 395–463 (1966).
Berner, R. A.: Principles of Chemical Sedimentology, McGraw-Hill Co., New York 1971.
Broderius, S. I., and Smith, L. L., Jr. Direct Determination and Calculation of Aqueous Hydrogen Sulfide Analyt. Chem.49 (3), 424–428 (1977).
Covington, A. (ed.): Ion-selective Electrode Methodology, vol. I, p. 239 CRC Press, Inc., Boca Raton 1979.
Frevert, T., and Galster, H.: A rapid and simple method for an in situ determination of Hydrogen Sulfide in water and sediments (in German), Schweiz. Z. Hydrol.40 (I), 199–208 (1978).
Gulens, J., and Ikeda, B.: Effects of Surface Heterogeneity on the Sensitivity of Sulfide Ion-Selective Electrodes. Analyt. Chem.50 (6), 782–787 (1978).
Long, F. A., and McDevit, W. F.: Activity Coefficients of Nonelectrolyte Solutes in Aqueous Salt Solutions. Chem. Rev.51, 119–169 (1952).
Randall M., and Failey, C. F.: The activity coefficient of non-electrolyte in aqueous salt solutions from solubility measurements, the salting out order of the ions; the activity coefficient of the undissociated part of weak electrolytes. Chem. Rev.4, 285–290 and 291–318 (1927).
Ross, I. W., Riseman, I. H., and Krueger, I. A.: Potentiometric Gas Sensing Electrodes. Pure appl. Chem.36, 473–486 (1973).
Steinhorn, I., Assaf, G., Gat, I. R., Nishry, A., Nissenbaum, N., Stiller, M., Beyth, M., Neev, D., Garber, R., Friedman, G. M., and Weiss, W.: The Dead Sea: Deepening of the Mixolimnion Signifies the Overture to Overturn of the Water Column. Science206, 55–57 (1979).
Stumm, W., and Morgan, J. J.: Aquatic Chemistry, p. 98, 104, Wiley-Interscience, New York 1970.
UNESCO: 2nd report of the joint panel on Oceanogrphic Tables and Standards. Paris 1966.
US-Standard Methods for the Examination of Water and Waste water, 14th ed., p. 503. APHA-AWWA-WPCF, Washington D.C. 1975.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Frevert, T. Determination of hydrogen sulfide in saline solutions. Schweiz. Z. Hydrologie 42, 255–268 (1980). https://doi.org/10.1007/BF02502437
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02502437