Abstract
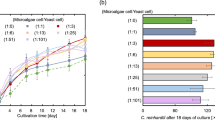
The freshwater green algaHaematococcus pluvialis (Strain Vischer 1923/2) grows best at high nitrate concentrations (about 0.5 to 1.0 g 1−1 KNO3), intermediate phosphate concentration (about 0.1 g 1−1 K2HPO4) and over a wide range of Fe concentrations. Low nitrate or high phosphate induce the formation of reddish palmella cells and aplanospores. Mixotrophic growth with acetate improves growth rate and final cell yield, and also stimulates the formation of the astaxanthin-containing palmella cells and aplanospores.H. pluvialis cannot grow above about 28 °C, or above a salinity of approximately 1% w/v NaCl. An increase in temperature or the addition of NaCl also stimulates the formation of palmella cells and aplanospores.
Similar content being viewed by others
References
Ben-Amotz A (1987) Effect of irradiance and nutrient deficiency on the chemical composition ofDunaliella bardawil Ben-Amotz and Avron (Volvocales, Chlorophyta). J. Plant Physiol. 3: 479–487.
Borowitzka MA (1988a) Vitamins and fine chemicals from micro-algae. In Borowitzka MA, Borowitzka LJ (eds), Micro-algal Biotechnology. Cambridge U.P., Cambridge, 153–196.
Borowitzka MA (1988b) Fats, oils and hydrocarbons. In Borowitzka MA, Borowitzka LJ (eds), Micro-algal Biotechnology, Cambridge U.P., Cambridge, 257–287.
Borowitzka MA, Borowitzka LJ (1988a)Dunaliella. In Borowitzka MA, Borowitzka LJ (eds), Micro-algal Biotechnology. Cambridge U.P., Cambridge, 27–58.
Borowitzka MA, Borowitzka LJ (1988b) Limits to growth and carotenogenesis in laboratory and large-scale outdoor cultures ofDunaliella salina. In Stadler T, Mollion J, Verdus MC, Karamanos Y, Morvan H, Christiaen D (eds), Algal Biotechnology. Elsevier Applied Science, London, 371–381.
Droop MR (1954) Conditions governing haematochrome formation inHaematococcus pluvialis Flotow. Arch. Mikrobiol. 20: 391–391.
Droop MR (1955) Carotenogenesis inHaematococcus pluvialis. Nature 175: 42.
Droop MR (1961)Haematococcus pluvialis and its allies. III. Organic nutrition. Rev. Algolog. 3: 247–259.
Elliot AM (1931) Morphology and life history ofHaematococcus pluvialis. Arch. Protistenk. 82: 250–272.
Endo H, Sansawa H, Nakajima K (1977) Studies onChlorella regularis, heterotrophic fast-growing strain. II. Mixotrophic growth in relation to light intensity and acetate concentration. Plant Cell Physiol. 18: 199–205.
Foss P, Storebakken T, Schiedt K, Liaaen-Jensen S, Austreng E, Streiff K (1984) Carotenoids in diets for salmonids. I. Pigmentation of rainbow trout with the individual optical isomers of astaxanthin in comparison with canthaxanthin. Aquaculture 41: 213–226.
Goldman JC, Dennet MR, Riley CB (1982) Effect of nitrogen-mediated changes in alkalinity on pH control and CO2 supply in intensive microalgal cultures. Biotech. Bioengng. 24: 619–631.
Goodwin TW, Jamikorn M (1954) Studies in carotenogenesis: II. Carotenoid synthesis in the algaHaematococcus pluvialis. Biochem. J. 57: 376–681.
Grung M, Bjerkeng B, Borowitzka MA, Skulberg O, Liaaen-Jensen S (1990) Alternative sources of astaxanthin, including secondary carotenoids of microalgae. Proc. 9th Internat. Symp. Carotenoids, Kyoto.
Jacobsen HC (1912) Kulturbedingungen vonHaematococcus pluvialis. Folia Microbiol. Delft, 1: 163.
Lwoff A, Lwoff M (1929) Le pouvoir de synthèse deChlamydomonas agloëformis etHaematococcus pluvialis en culture pure à l'obscurité. C.R. Soc. Biol. Paris, 102: 569–571.
Piorreck M, Baasch K-H, Pohl P (1984) Biomass production, total protein, chlorophylls, lipids and fatty acids of freshwater and blue-green algae under different nitrogen regimes. Phytochemistry 23: 207–216.
Pringsheim EG (1914) Kulturversuche mit chlorophyllführenden Mikroorganismen. IV. Die Ernährung vonHaematococcus pluvialis Flot. Beitr. Biol. Pflanz. 11: 305–332.
Pringsheim EG (1966) Nutritional requirements ofHaematococcus pluvialis and related species. J. Phycol. 2: 1–7.
Proctor VW (1957) Preferential assimilation of nitrate iron byHaematococcus pluvialis. Am. J. Bot. 44: 141–143.
Renstrøm B, Borch G, Skulberg OM, Liaaen-Jensen S (1981) Optical purity of (3S,3S′)-astaxanthin fromHaematococcus pluvialis. Phytochem. 20: 2561–2564.
Santos MF, Mesquita JF (1984) Ultrastructural study ofHaematococcus lacustris (Girad.) Rostafinski (Volvocales). I. Some aspects of carotenogenesis. Cytologie 49: 215–228.
Semenko VE, Abdullayev AA (1980) Parametric control of β-carotene biosynthesis inDunaliella salina cells under conditions of intensive cultivation. Fiziol. Rasten. 27: 31–41.
Sommer TR, Potts WT, Morrisey NM (1991) Utilization of microalgal astaxanthin by rainbow trout (Oncorhynchus mykiss). Aquaculture 94: 79–88.
Sprey B (1970) Die Lokalisierung von Sekundarcarotinoiden vonHaematococcus pluvialis Flowtow emen. Wille. Protoplasma 71: 235–250.
Storebakken T, Foss P, Schiedt K, Austreng E, Liaaen-Jensen S, Manz U (1987) Carotenoid diets in salmonids. IV. Pigmentation of Atlantic salmon with astaxanthin, astaxanthin dipalmitate and canthaxanthin. Aquaculture 65: 279–292.
Stross RG (1963) Nitrate preference inHaematococcus pluvialis is controlled by strain, age of inoculum and pH of the medium. Can. J. Microbiol. 9: 33–40.
Syrett PJ (1962) Nitrogen assimilation. In Lewin RA (ed.), Physiology and Biochemistry of Algae. Academic Press, London, 171–188.
Viala G (1966) L'astaxanthin chez leChlamydomonas nivalis Wille. C.R. Acad. Sci. Paris D. 263: 1383–1386.
Wiessner W (1979) Photoassimilation of organic compounds. In Gibbs M, Latzko E (eds), Encyclopedia of Plant Physiology, New Series, Vol. 6. Springer Verlag, Berlin, 181–189.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Borowitzka, M.A., Huisman, J.M. & Osborn, A. Culture of the astaxanthin-producing green algaHaematococcus pluvialis 1. Effects of nutrients on growth and cell type. J Appl Phycol 3, 295–304 (1991). https://doi.org/10.1007/BF02392882
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02392882