Abstract
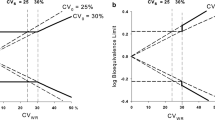
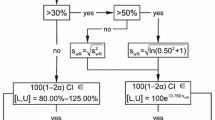
Recent work, beginning with that of Anderson and Hauck in 1990, has led to a general acceptance of the need to ensure switchability in bioequivalence testing for approval of generic drugs. In other applications of bioequivalence testing, prescribability may be sufficient. However, there is less acceptance of the need to change statistical procedures and study designs from those currently used to assess the current criterion of average bioequivalence. We propose easily interpreted measures of switchability and prescribability. These measures provide bases for assessing conditions under which average bioequivalence is not sufficient to ensure switchability and prescribability, and hence for which a procedure for individual or population bioequivalence is required. The required conditions are sufficiently tight that they cannot be presumed to hold. Thus, there are reasonable conditions for which current practice is not sufficient. An outcome of this development is a connection between two current approaches for assessing individual bioequivalence.
Similar content being viewed by others
References
S. Hwang, P. B. Huber, M. Hesney, and K. C. Kwan. Bioequivalence and interchangeability.J. Pharm. Sci. 67: June Open Form, page iv (1978).
S. Anderson and W. W. Hauck. Consideration of individual bioequivalence.J. Pharmacokin. Biopharm. 18:259–273 (1990).
W. W. Hauck and S. Anderson. Types of bioequivalence and related statistical considerations.Int. J. Clin. Pharm. Ther. Toxicol. 30:181–187 (1992).
J. D. Esinhart and V. M. Chinchilli. Extension to the use of tolerance intervals for the assessment of individual bioequivalence.J. Biopharm. Statist. 4:39–52 (1994).
R. Schall. Assessment of individual and population bioequivalence using the probability that bioavailabilities are similar.Biometrics (in press).
L. B. Sheiner. Bioequivalence revisited.Statist. Med. 11:1777–1788 (1992).
G. Ekbohm and H. Melander. The subject-by-formulation interaction as a criterion of interchangeability of drugs.Biometrics 45:1249–1254 (1989).
R. Schall and H. G. Luus. On population and individual bioequivalence.Statist. Med. 12:1109–1124 (1993).
D. J. Holder and F. Hsuan. Moment-based criteria for determining bioequivalence.Biometrika 80:835–846 (1993).
L. Endrenyl. A method for the evaluation of individual bioequivalence.Int. J. Clin. Pharm. Ther. Toxicol. 32:497–508 (1994).
W. W. Hauck and S. Anderson. The transitivity of bioequivalence testing: Potential for drift. Submitted, 1994.
D. J. Schuirmann. A comparison of the two one-sided test procedure and the power approach for assessing the bioequivalence of average bioavailability.J. Pharmacokin. Biopharm. 15:657–680 (1987).
Author information
Authors and Affiliations
Additional information
Supported in part by a grant from the National Heart, Lung, and Blood Institute (No HL51401).
Rights and permissions
About this article
Cite this article
Hauck, W.W., Anderson, S. Measuring switchability and prescribability: When is average bioequivalence sufficient?. Journal of Pharmacokinetics and Biopharmaceutics 22, 551–564 (1994). https://doi.org/10.1007/BF02353794
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/BF02353794