Abstract
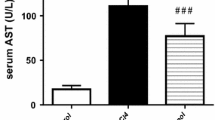
In this study, the intraperitoneal administration of 1 mg/kg thioacetamide (TAA) produced hepatotoxicity in mice. The increase in serum SGOT and SGPT produced at 24 h by this regimen was decreased in a dose-dependent manner by coadministration of tetramethylpyrazine (TMP; 10, 25 and 50 mg/kg). A rise in serum interleukin-2 was similarly prevented. Increased concentrations of malondialdehyde (MDA) generated in vitro in liver homogenates prepared from TAA-treated mice were decreased by TMP treatments. The increase in MDA produced by TAA was also prevented by in vitro addition of TMP to liver homogenates. These results suggest that part of the hepatocellular injury induced by TAA is mediated by oxidative stress caused by the action of cytokines through lipid peroxidation. TMP appears to act by preventing lipid peroxidation.
Similar content being viewed by others
References
Adamson GM, Billings RE. Cytokine toxicity and induction of NO synthase activity in cultured mouse hepatocytes. Toxicol Appl Pharmacol 119:100–107;1993.
Akbay A, Cinar K, Uzunalimoglu O, Eranil S, Yurdaydin C, Bozkaya H, Bozdayi M. Serum cytotoxin and oxidant stress markers in N-ace-tylcysteine treated thioacetamide hepatotoxicity of rats. Hum Exp Toxicol 18:669–676;1999.
Anghileri LJ, Heidbreder M, Weiler G, Dermietzel R. Hepatocarcinogenesis by thioacetamide: Correlations of histological and biochemical changes, and possible role of cell injury. Exp Cell Biol 45:34–47;1977.
Bacon BR, Tavill AS, Brittenham GM, Park CH, Recknagel RO. Hepatic lipid peroxidation in vivo in rats with chronic iron overload. J Clin Invest 71:429–439;1983.
Bozkaya H, Bozdayi AM, Aslan N, Turkay C, Sarioglu M, Cetinkaya H, Akdogan M, Cinar K, Erden E, Kose K, Senturk H, Akkiz H, Karayalcin S, Yurdaydin C, Uzunalimoglu O. Circulating IL-2 and IL-10 in chronic active hepatitis C with respect to the response to IFN treatment. Infection 28:309–313;2000.
Bruck R, Aeed H, Shirin H, Matas Z, Zaidel L, Avni Y, Halpern Z. The hydroxyl radical scavengers dimethylsulfoxide and dimethylthiourea protect rats against thioacetamide-induced fulminant hepatic failure. J Hepatol 31:27–38;1999.
Bruno MK, Cohen SD, Khairallah EA. Antidotal effectiveness of N-acetylcysteine in reversing acetaminophen-induced hepatotoxicity. Biochem Pharmacol 37:4319–4325;1988.
Cascales M, Martin-Sanz P, Craciunescu DG, Mayo I, Aguilar A, Robles-Chillida EM, Cascales C. Alterations in hepatic peroxidation mechanisms in thioacetamide-induced tumors in rats. Effects of a rhodium(III) complex. Carcinogenesis 12:233–240;1991.
Chieli E, Malvaldi G. Role of the microsomal FAD-containing monooxygenase in the liver toxicity of thioacetamide S-oxide. Toxicology 31:41–51;1984.
Faa G, Ambu R, Congiu T, Costa V, Ledda-Columbano GM, Coni P, Curto M, Giacomini L, Columbano A. Early ultrastructural changes during thioacetamide-induced apoptosis in rat liver. J Submicrose Cytol Pathol 24:417–424;1992.
Hill DB, Marsano LS, McClain CJ. Increased plasma interleukin-8 concentrations in alcoholic hepatitis. Hepatology 18:576–580;1993.
Khoruts A, Stahnke L, McClain CL, Logan G, Allen J. Circulating tumor necrosis factor, interleukin-1 and interleukin-6 concentrations in chronic alcoholic patients. Hepatology 13:267–276;1991.
Liao MH, Wu CC, Yen MH. Beneficial effects of tetramethylpyrazine, an active constituent of Chinese herbs, on rats with endotoxemia. Proc Natl Sci Counc Repub China B 22:46–54;1998.
Liu SL, Degli Esposti S, Yao T, Diehl AM, Zern MA. Vitamin E therapy of acute CCl4-induced hepatic injury in mice is associated with inhibition of nuclear factor kappa B binding. Hepatology 22:1474–1481;1995.
Meyer M, Caselmann WH, Schluter V, Schreck R, Hofschneider PH, Baeuerle PA. Hepatitis B virus transactivator MHBst: Activation of NFκB, selective inhibition of antioxidants and integral membrane localization. EMBO J 11:2991–3001;1992.
Moreira E, Fontana L, Periago JL, Sanchez De Medina F, Gil A. Changes in fatty acid composition of plasma, liver microsomes, and erythrocytes in liver cirrhosis induced by oral intake of thioacetamide in rats. Hepatology 21:199–206;1995.
Rubio N, Torres C. IL-1, IL-2 and IFN-gamma production by Theiler's virus-induced enceph-alomyelitic SJL/J mice. Immunology 74:284–289;1991.
Sanz N, Diez-Fernandez C, Fernandez-Simon L, Alvarez A, Cascales M. Necrogenic and regenerative responses of liver of newly weaned rats against a sublethal dose of thioacetamide. Biochim Biophys Acta 1384:66–78;1998.
Shieh YH, Liu CF, Huang YK, Yang JY, Wu IL, Lin CH, Lin SC. Evaluation of the hepatic and renal-protective effects ofGanodermal lucidum in mice. Am J Chin Med 29:501–507;2001.
Slater TF. Free radical mechanism in tissue injury. Biochem J 222:1–15;1984.
Torres MI, Fernandez MI, Gil A, Rios A. Dietary nucleotides have cytoprotective properties in rat liver damaged by thioacetamide. Life Sci 62:13–22;1998.
Vogl S, Junker U, Vogelsang H, Dargel R. Macrophages from rat livers with micronodular and macronodular cirrhosis differ with respect to mediator release and DNA-synthesis. J Hepatol 26:1093–1103;1997.
Wong SH, Knight JA, Hopfer SM, Zaharia O, Leach CNJr, Sunderman FWJr. Lipoperoxides in plasma as measured by lipid-chromatographic separation of malondiadehyde-thiobarbituric acid adduct. Clin Chem 33:214–220;1987.
Yamashiki M, Mase A, Arai I, Huang XX, Nobori T, Nishimura A, Sakaguchi S, Inoue K. Effects of the Japanese herbal medicine ‘Inchinko-to’ (TJ-135) on concanavalin A-induced hepatitis in mice. Clin Sci (Lond) 99:421–431;2000.
Yuda Y, Tanaka J, Hirano F, Igarashi K, Statoh T. Participation of lipid peroxidation in rat pertussis vaccine pleurisy. 3. Thiobarbituric acid (TBA) reactant and lysosomal enzyme. Chem Pharm Bull (Tokyo) 39:505–506;1991.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
So, E.C., Wong, KL., Huang, TC. et al. Tetramethylpyrazine protects mice against thioacetamide-induced acute hepatotoxicity. J Biomed Sci 9, 410–414 (2002). https://doi.org/10.1007/BF02256534
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02256534