Abstract
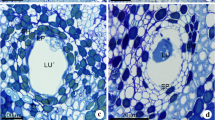
The occurrence and ultrastructure of eubacteria within leaf cavities of symbiotic and cyanobiont-freeAzolla mexicana were examined with transmission electron microscopy. Bacteria were observed in all leaf cavities of bothAzolla cultures, showing increasing numbers concomitant with leaf age. In symbioticAzolla mexicana two ultrastructurally distinct types of bacteria were found; a helical bacterium and a slightly curved, rod-shaped bacterium. Both had typical Gram-negative cell wall ultrastructure. The helical bacteria comprised 60% of the total population in young leaf cavities (1–5) and 74–80% of the population in older cavities. The mode of cell division for the rod-shaped bacterium was binary fission. In cyanobiont-freeAzolla mexicana at least three distinct types of bacteria were observed; two were ultrastructurally similar to, if not identical, with the types present in symbioticAzolla mexicana.
Similar content being viewed by others
Literature Cited
Beveridge TJ (1981) Ultrastructure, chemistry, and function of the bacterial wall. Int Rev Cytol 72:229–317
Bottomley WB (1920) The effect of organic matter on the growth of various water plants in culture solution. Ann Bot 39:353–365
Boylen CW, Pate JL (1973) Fine structure ofArthrobacter crystallopoietes during long-term starvation of rod and spherical stage cells. Can J Microbiol 19:1–5
Braun-Howland EB, Lindblad P, Nierzwicki-Bauer SA, Bergman B (1988) Dinitrogenase reductase (Fe-protein) in the cyanobacterial symbionts of threeAzolla species: localization and sequence of appearance during heterocyst differentiation. Planta 176:319–332
Caiola MG, Forni C, Castagnola M (1988) Bacteria in theAzolla-Anabaena association. Symbiosis 5:185–198
Chao L, Bowen CC (1971) Purification and properties of glycogen isolated from a blue-green alga,Nostoc muscorum. J Bacteriol 105:331–338
Gates JE, Fisher RW, Candler RA (1980) The occurrence of coryneform bacteria in the leaf cavity ofAzolla. Arch Microbiol 127:163–165
Krulwich TA, Pate JL (1971) Ultrastructural explanation for snapping postfission movements inArthrobacter crystallopoietes. J Bacteriol 105:408–412
Kvasnikov EI, Pisarchuk EN, Ivanova LL, Gushcha KP, Andrienko VI (1986) The taxonomic significance of cell division and separation types inArthrobacter species. Mikrobiologiya 55:662–668
Luscombe BM, Gray TRG (1971) Effect of varying growth rate on the morphology ofArthrobacter. J Gen Microbiol 69:433–434
Newton JW, Herman AI (1979) Isolation of cyanobacteria from the aquatic fern,Azolla. Arch Microbiol 120:161–165
Peters GA (1977) TheAzolla-Anabaena azollae symbiosis. In: Hollaender A (ed) Genetic engineering for nitrogen fixation. New York: Plenum Publishing Corp, pp 231–258
Peters GA, Mayne BC (1974) TheAzolla, Anabaena azollae relationship I. Initial characterization of the association. Plant Physiol 53:813–819
Peters GA, Mayne BC (1974) TheAzolla, Anabaena azollae relationship II. Localization of nitrogenase activity as assayed by acetylene reduction. Plant Physiol 53:820–824
Peters GA, Ray TB, Mayne BC, Toia RE Jr (1980)Azolla-Anabaena association: morphological and physiological studies. In: Newton WE, Orme-Johnson WH (eds) Nitrogen fixation, vol 2. Baltimore: University Park Press, pp 293–309
Peters GA, Toia RE Jr, Evans WR, Crist DK, Mayne BC, Poole RE (1980) Characterization and comparisons of five N2-fixingAzolla-Anabaena associations. I. Optimization of growth conditions for biomass increase and N content in a controlled environment. Plant Cell Env 3:261–269
Petro MJ, Gates JE (1987) Distribution ofArthrobacter sp. in the leaf cavities of four species of the N-fixingAzolla fern. Symbiosis 3:41–48
Ray TB, Peters GA, Mayne BC, Toia RE Jr (1978)Azolla-Anabaena relationship. VII. Distribution of ammonia-assimilating enzymes, protein, and chlorophyll between host and symbiont. Plant Physiol 62:463–467
Ray TB, Mayne BC, Toia RE Jr, Peters GA (1979)Azolla-Anabaena relationship. VIII. Photosynthetic characterization of the association and individual partners. Plant Physiol 64:791–795
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Shively JM (1974) Inclusion bodies of prokaryotes. Annu Rev Microbiol 28:167–187
Spurr AR (1969) A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res 26:31–43
Stevens SE Jr, Paone DAM, Balkwill DL (1981) Accumulation of cyanophycin granules as a result of phosphate limitation inAgmenellum quadruplicatum. Plant Physiol 67:716–719
Wallace WH, Gates JE (1986) Identification of eubacteria isolated from leaf cavities of four species of the N-fixingAzolla fern asArthrobacter Conn and Dimmick. Appl Environ Microbiol 52:425–429
Watanabe I, Espiñas CR, Berja NA, Alimagno BV (1977) The utilization of theAzolla-Anabaena complex as a nitrogen fertilizer for rice. IRRI Res Paper Series 11:1–15
Ward CM Jr, Claus GW (1973) Gram characteristics and wall ultrastructure ofArthrobacter crystallopoietes during coccus-rod morphogenesis. J Bacteriol 114:378–389
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Nierzwicki-Bauer, S.A., Aulfinger, H. Ultrastructural characterization of eubacteria residing within leaf cavities of symbiotic and cyanobiont-freeAzolla mexicana . Current Microbiology 21, 123–129 (1990). https://doi.org/10.1007/BF02091830
Issue Date:
DOI: https://doi.org/10.1007/BF02091830