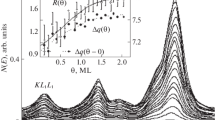
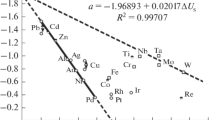
Abstract
The interaction of adsorbates with metal surfaces is discussed. It is shown that the evanescent charge density produced by occupied sp derived surface states yields a considerable contribution to the Pauli repulsion experienced by adsorbate particles with a closed-shell electronic structure, e.g. rare-gases or molecules such as H2 or N2. For rare-gases this results in a reduction of the binding energy in the presence of occupied surface states, for molecules this gives rise to an additional contribution to the dissociation barrier. Suitable surface dopants are able to depopulate surface states and thereby to reduce the dissociation barrier. Such dopants can substantially promote catalytic reactions in which the dissociation from the gas phase or a physisorbed precursor is the rate limiting step. In contrast to closed-shell systems the bonding interaction for metal adsorbates on metal substrates is enhanced by occupied surface states. This leads to an extra diffusion barrier at steps, because the surface state amplitude drops to zero at upper step edges. The additional step-edge barrier, which is a kinetic hindrance for layer-by-layer growth, can be reduced by surface dopants depopulating the corresponding surface state. Such dopants promote layer-by-layer growth and act therefore as surfactants. It is concluded that the effect of promoters in catalysis and of surfactants in metal epitaxy is in part due to the same basic mechanism, namely the depopulation of surface states.
Similar content being viewed by others
References
M.P. Kiskinova: Poisoning and Promotion in Catalysis Based on Surface Science Concepts and Experiments, Elsevier, Amsterdam (1993)
H.P. Bonzel: Surf. Sci. Rep.8, 43 (1987)
J.E. Müller:Theory of the Coadsorption of H 2 O and CO with K on the Pt(111) Surface, in: The Chemical Physics of Solid Surfaces, Vol. 6, ed. D.A. King and D.P. Woodruff, Elsevier, Amsterdam (1993) p 29
P.A. Schultz, Ch.H. Patterson, R.P. Messmer: J. Vac. Sci. Technol.A 5, 1061 (1986)
K. Wandelt,Atomic Scale Surface Characterization with Photoemission of Adsorbed Xenon (PAX ), in: Chemistry and Physics of Solid Surfaces, Vol. 8, eds. R. Vanselow and R. Howe, Springer Series in Surface Science22, Springer Verlag, Berlin (1990) p 289
T.V.W. Janssens, G.R. Castro, K. Wandelt, J.W. Niemantsverdriet: Phys. Rev. B49, 14599 (1994)
P.J. Feibelman, D.R. Hamann: Surf. Sci.149, 48 (1985)
M. Henzler: Prog. Surf. Sci.42, 297 (1993)
G. Rosenfeld, N.N. Lipkin, W. Wulfhekel, J. Kliewer, K. Morgenstern, B. Poelsema, G. Comsa:Appl. Phys. A61, 455 (1995)
R.L. Schwoebel, E.J. Shipsey: J. Appl. Phys.37, 3682 (1966)
G. Ehrlich, F.G. Hudda: J. Chem. Phys.44, 1039 (1966)
G. Rosenfeld, R. Servaty, C. Teichert, B. Poelsema, G. Comsa: Phys. Rev. Lett.71, 895 (1993)
S. Oppo, V. Fiorentini, M. Scheffler: Phys. Rev. Lett.71, 2437 (1993)
St. Esch, M. Hohage, Th. Michely, G. Comsa: Phys. Rev. Lett.72, 518 (1994)
N. Memmel, E. Bertel: Phys. Rev. Lett.75, 485 (1995)
J. Harris, A. Liebsch: J. Phys. C15, 2275 (1982)
P. Roos, E. Bertel, K.D. Rendulic: Chem. Phys. Lett.232, 537 (1995)
E. Bertel: Surf. Sci.331-333, 1136 (1995)
E. Zaremba; W. Kohn: Phys. Rev. B15, 1769 (1977)
K. Wandelt, B. Gumhalter: Surf. Sci.140, 355 (1984)
M. Wolf, E. Knoesel, T. Hertel: Phys. Rev. B Rapid Commun., in print
W.R. Merry, R.E. Jordan, D.F. Padowitz, C.B. Harris: Surf. Sci.295, 393 (1992)
K. Kern, R. David, R.L. Palmer, G. Comsa: Surf. Sci.175, L669 (1986)
M. Potthoff, G. Hilgers, N. Miller, U. Heinzmann, L. Haunert, J. Braun, G. Borstel: Surf. Sci.322, 193 (1995); G. Hilgers, M. Potthoff, N. Müller, U. Heinzmann: Surf. Sci.322, 207 (1995)
M. Petersen, S. Wilke, P. Ruggerone, B. Kohler, M. ScheflJer: Phys. Rev. Lett.76, 995 (1996);d states do play an important role, because in Pd and Pt they provide a large DOS atE F and therefore the charge driven back into the metal is mainly accommodated into thed states. The surface states or resonances which are responsible for the repulsive interaction are in generalspd hybride states, but the overlap with the rare-gas atom is essentially due to thesp admixture in the wavefunction
S.D. Kevan, R.H. Gaylord: Phys. Rev. B36, 5809 (1987)
E. Bertel: Surf. Sci. Lett., in print
J. Tersoff, D.R. Hamann: Phys. Rev. B31, 805 (1985)
S. Horch, P. Zeppenfeld, G. Comsa: Appl. Phys. A60, 147 (1995)
K. Wandelt, J.E. Hulse: J. Chem. Phys.80, 1340 (1984)
P. Zeppenfeld, S. Horch, G. Comsa: Phys. Rev. Lett.73, 1259 (1994)
B. Hammer, J.K. Nørskov: Surf. Sci.343, 211 (1995)
P.M. Holmblad, J. Hvolbæk Larsen, I. Chorkendorff, L. Pleth Nielsen, F. Besenbacher, I. Stensgaard, E. Lægsgaard, P. Kratzer, B. Hammer, J.K. Nørskov: Catal. Lett.40, 131 (1996)
M. Bowker,Promotion in Ammonia Synthesis, in: The Chemical Physics of Solid Surfaces, Vol. 6, eds. D.A. King, D.P. Woodruff: Elsevier, Amsterdam (1993) p 225
Ch. Resch, H.F. Berger, K.D. Rendulic, E. Bertel: Surf. Sci. Lett.316, L1105 (1994)
N. Memmel, G. Rangelov, E. Bertel, V. Dose: Phys. Rev. B43, 6938 (1991)
P. Sandl, E. Bertel: Surf. Sci. Lett.302, L325 (1994)
G.M. Watson, P.A. Bruhwiler, E.W. Plummer, H.-J. Sagner, K.-H. Frank: Phys. Rev. Lett.65, 468 (1990)
N. Fischer, S. Schuppler, Th. Fauster, W. Steinmann: Surf. Sci.314, 89 (1994)
Ch. Resch, V. Zhukov, A. Lugstein, H.F. Berger, A. Winkler, K.D. Rendulic: Chem. Phys.177, 421 (1993)
J.K. Brown, A.C. Luntz, P.A. Schultz: J. Chem. Phys.95, 3767 (1991)
E. Bertel, P. Roos, J. Lehmann: Phys. Rev. B52, R14384 (1995)
E. Bertel, P. Sandl, K.D. Rendulic, M. Beutl: Ber. Bunsenges. Phys. Chem.100, 114 (1996)
F. Passek, M. Donath: Phys. Rev. Lett.71, 2122 (1993)
S.D. Kevan, N.G. Stoffel, N.V. Smith: Phys. Rev. B31, 3348 (1985)
N.V. Smith, C.T. Chen: Surf. Sci.247, 133 (1991)
E. Bertel: Phys. Rev. B50, 4925 (1994)
R.J. Behm:Alkali Adsorption and Alkali induced Reconstructions on fcc(1 10) Metal Surfaces, in: Physics and Chemistry of Alkali Metal Adsorption, eds. H.P. Bonzel, A.M. Bradshaw, G. Ertl, Materials Science Monographs57, Elsevier, Amsterdam (1989) 111
E. Bertel:Surface States and Chemical Reactivity of Metals, in: Electronic Surface and Interface States on Metallic Systems, eds. E. Bertel and M. Donath, World Scientific, Singapore (1995) p 13
P. Kratzer, B. Hammer, J.K. Nørskov: Surf. Sci.359, 45 (1996)
R. Matzdorf, R. Paniago, G. Meister, A. Goldmann: Solid State Commun.96, 799 (1995)
J.K. Nørskov:Adsorbate-Adsorbate Interactions on Metal Surfaces, in: The Chemical Physics of Solid Surfaces, Vol. 6, eds. D.A. King and D.P. Woodruff, Elsevier, Amsterdam (1993) p 1
G. Ertl: Catal. Rev.-Sci. Eng.21, 201 (1980)
L.J. Whitman, C.E. Bartosch, W. Ho, G. Strasser, M. Grunze: Phys. Rev. Lett.56, 1984 (1986)
L.J. Whitman, C.E. Bartosch, W. Ho: J. Chem. Phys.85, 3688 (1986)
Inspection of the Fe band structure reveals ap-sd bandgap atE F in the minority bands above the N1′ point. On Fe(1 10) the N1′ wavefunction projects as ap z type wavefunction onto the center of the SBZ and has therefore a large decay length. More precisely, with the N1 wavefunction it will form a\(p_z d_{z^2 } \) hybride surface state. This state dominates the charge density at larger distances from the surface. On the Fe(100) surface the N1′ point projects onto the edges of the SBZ. The corresponding surface state has nodal planes going through the atoms. The same applies to the Fe(111) surface
See for instance the references cited in ref. 42
W.C.A.N. Ceelen, A.W. Denier van der Gon, J. Falta, A. Hille, G. Materlik: Verhandl. DPG (VI)31, 034.5 (1996)
H.A. van der Vegt, H.M. van Pixteren, M. Lohmeier, E. Vlieg: Phys. Rev. Lett.68, 3335 (1992)
R. Kunkel, B. Poelsema, L.K. Verheij, G. Comsa: Phys. Rev. Lett.65, 733 (1990)
J.K. Nørskov: Rep. Prog. Phys.53, 1253 (1990)
H. Wolter, M. Schmidt, K. Wandelt: Surf. Sci.298, 173 (1993); M. Schmidt, H. Wolter, K. Wandelt: Surf. Sci.307-309, 507 (1994)