Abstract
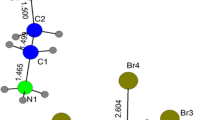
Raman spectroscopy on solid anhydrous sodium dithionite reveals that it exists in at least two forms in the solid state. MAS23Na NMR spectra and X-ray powder diffraction patterns of the solids indicate that sodium ions are in different environments in each form of the material. The results suggest that the dithionite anion is conformationally different in each lattice. A single crystal X-ray diffraction study of the recrystallized form of anhydrous sodium dithionite reveals an anion with C2 geometry and a 16° O-S-S-O torsional angle; nearly eclipsed. (Crystal Data for recrystallized Na2S2O4 area=6.539(1) Å,b=6.552(1) Å,c=6.578(1) Å,V=240.0(1) Å3,β=121.61(1)°, space group=P2/c,Z=2). Raman spectra of sodium dithionite dihydrate reveal that the dithionite ion is in a different conformation than in either of the anhydrous materials. A single crystal X-ray diffraction study of Na2S2O4·2H2O reveals a dithionite anion with a substantially shorter S-S bond length than in the anhydrous structure and an O-S-S-O torsional angle of 56°; approximately gauche. (Crystal Data for Na2S2O4·2H2O area=8.134(1) Å,b=5.756(2) Å,c=14.528(5) Å,V=653.3(3) Å3,β=106.20(2)°, space group=P21/n,Z=4). The structure of the dithionite anion is found to depend critically upon the nature of its external environment.
Similar content being viewed by others
References
Berzelius, J. J. (1825) Lehrbuch der Chemie, Dresden, p. 472.
Bennett, D. W. (1990) Locally written IBM PC/AT-based data collection software.
Cotton, F. A., and Wilkinson, G. (1988)Advanced Inorganic Chemistry (fifth edition) (Wiley, New York), p. 523.
Cromer, D. T. (1974)International Tables for X-ray Crystallography (Kynoch Press, Birmingham, England), Vol IV.
Dunitz, J. D. (1956)Acta Crystallogr. 9, 579.
Goodhead, K., O'Neill, I., and Wardleworth, D. (1971)J. Appl. Chem. Biotech. 24, 71.
Hamilton, W. C., and Ibers, J. A. (1968)Hydrogen Bonding in Solids (W. A. Benjamin, Inc., New York), pp. 14–18.
Hodgeman, W. C., Weinrach, J. B., and Bennett, D. W. (1991)Inorg. Chem. 30, 1611.
Jellinek, J. Z. (1911)Anorg. Allg. Chem. 70, 93.
Kiers, C. T., and Vos, A. (1978)Acta Crystallogr. B 34, 1499.
Kirfel, A., and Will, G. (1980)Acta Crystallogr. B 36, 223.
Kubas, G. J., Wasserman, H. J., and Ryan, R. R. (1985)Organometallics 4, 2012.
Leszczynski, J., and Zerner, M. C. (1989)Chem. Phys. Lett. 159, 143.
Lyons, D., and Nickless, G. (1968)Inorganic Sulfur Chemistry (Elsevier, New York), pp. 519–533.
Magnusson, A., and Johansson, L. (1982)Acta Chem. Scand. A 36, 429.
Olfield, E., Schramm, S., Meadows, M. D., Smith, K. A., Kinsey, R. A., and Ackerman, J. (1982)J. Am. Chem. Soc. 104, 919.
Peter, L., and Meyer, B. (1982)J. Mol. Struct. 95, 131.
Rietveld, H. M. (1967)Acta Crystallogrs. 22, 151.
Scholder, M., and Denk, G. Z. (1935)Anorg. Allg. Chem. 222, 41.
Sheldrick, G. M. (1976)Shelx-76.A program for crystal structure solution. (Institute fur Anorganische Chemie der Universitat, Gottingen, Federal Republic of Germany).
Sheldrick, G. M. (1986)Shelxs-86.A program for crystal structure solution. (Institute fur Anorganische Chemie der Universitat, Gottingen, Federal Republic of Germany).
Simon, V. A., and Kuchler, H. Z. (1949)Anorg. Chem. 260, 161.
Takashashi, H., Kaneko, N., and Miwa, K. (1982)Spectrochim. Acta 38A, 1147.
Touzain, P., and Ayedi, F. (1972)C. R. Acad. Sci. Paris 274, 1911.
Weinrach, J. B., and Bennett, D. W. (1991)J. Appl. Cryst. 24, 91.
Wilson, A. J. C. (1942)Nature (London) 150, 151.
Yost, D. M. (1946) Russel, H. (1946)Systematic Inorganic Chemistry (Prentice-Hall, New York), pp. 354–357.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Weinrach, J.B., Meyer, D.R., Guy, J.T. et al. A structural study of sodium dithionite and its ephemeral dihydrate: A new conformation for the dithionite ion. Journal of Crystallographic and Spectroscopic Research 22, 291–301 (1992). https://doi.org/10.1007/BF01199531
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01199531