Abstract
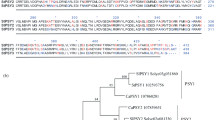
A cDNA clone encoding mitochondrial citrate synthase (EC 4.1.3.7), the first enzyme of the tricarboxylic-acid cycle, was isolated from potato (Solanum tuberosum L.) and expression of the enzyme analyzed. The deduced amino-acid sequence of the potato mitochondrial citrate synthase showed high similarity to known citrate synthases from fungi, mammals andArabidopsis thaliana. The expression pattern of this clone was determined by Northern blot analysis. Expression was detected in all tissues analyzed. The highest level of expression was found in green flower buds. In photosynthetic tissues, stronger mRNA expression was detected in mature than in immature leaves. This rise in expression with leaf age was accompanied by an increase in citrate-synthase activity. Within flowers, expression was severalfold stronger in anthers than in ovaries, indicating a role of mitochondrial citrate synthase during anther or pollen development. A comparatively low level of transcript was detected in underground heterotrophic tissues, such as stolons, tubers and roots. When tubers were stored at low temperature (4°C), mitochondrial citrate-synthase gene expression increased slightly. From the data obtained, we conclude that expression of the mitochondrial citrate-synthase gene is regulated by developmental and environmental factors. The relatively high expression in leaves is in line with the assumption that mitochondria play an important role in photosynthetically active tissues.
Similar content being viewed by others
Abbreviations
- PCR:
-
polymerase chain reaction
References
Ap Rees, T. (1990) Carbon metabolism in mitochondria. In: Plant physiology, biochemistry and molecular biology, pp. 106–123, Dennis, D.T., Turpin, D.H., eds. Longman Scientific and Technical, Longman, Singapore
Bell, A.W., Bhayana, V., Duckworth, H.W. (1983) Evidence for structural homology between the subunits from allosteric and nonallosteric citrate synthase. Biochemistry22, 3400–3405
Bloxham, D.P., Parmelee, D.C., Kumar, S., Walsh, K.A., Titani, K. (1982) Complete amino acid sequence of porcine heart citrate synthase. Biochemistry21, 2028–2036
Bradford, M.M. (1976) Rapid and quantitative method for quantitation of microgram quantities of protein utilizing the principle of proteindye binding. Anal. Biochem.72, 248–252
Colas de Francs-Small, C., Ambard-Bretteville, F., Darpas, A., Sallantin, M., Huet, J.-C., Rémy, R. (1992) Variation of the polypeptide composition of mitochondria isolated from different potato tissues. Plant Physiol.98, 273–278
Devereux, J., Haeberli, P., Smithies, O. (1984) A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res.12, 387–395
Douce, R., Neuburger, M. (1989) The uniqueness of plant mitochondria. Annu. Rev. Plant Physiol. Plant Mol. Biol.40, 371–414
Dry, I.B., Wiskich, J.T. (1986) Interactions of mitochondria with other metabolic processes-an overview. In: Plant mitochondria: Structural, functional and physiological aspects, pp. 151–160, Moore, A.L., Beechey, R.B., eds. Plenum Press, New York
Evans, C.T., Owens, D.D., Sumegi, B., Kispal, G., Srere, P.A. (1988) Isolation, nucleotide sequence, and expression of a cDNA encoding pig citrate synthase. Biochemistry27, 4680–4686
Hanna, Z., Fregeau, C., Préfontaine, G., Brousseau, R. (1984) Construction of a family of universal expression plasmid vectors. Gene30, 247–250
Hanning, I., Heldt, H.W. (1993) On the function of mitochondrial metabolism during photosynthesis in spinach (Spinacia oleracea L.) leaves. Plant Physiol.103, 1147–1154
Hanson, M.R. (1991) Plant mitochondrial mutations and male sterility. Annu. Rev. Genet.25, 461–486
Hanson, M.R., Conde, M.F. (1985) Functioning and variation of cytoplasmic genomes: Lessons from cytoplasmic-nuclear interactions affecting male fertility in plants. Int. Rev. Cytol.94, 213–267
Hartl, F.-U, Pfanner, N., Nicholson, D.W., Neupert, W. (1989) Mitochondrial protein import. Biochim. Biophys. Acta988, 1–45
Havelange, A. (1980) The quantitative ultrastructure of the meristematic cells ofXanthium strumarium during the transition to flowering. Am. J. Bot.67, 1171–1178
Huang, J., Struck, F., Matzinger, D.F., Levings III, C.S. (1994) Flowerenhanced expression of a nuclear-encoded mitochondrial respiratory protein is associated with changes in mitochondrion number. Plant Cell6, 439–448
Joshi, C.P. (1987) Putative polyadenylation signals in nuclear genes of higher plants: a compilation and analysis. Nucleic Acids Res.15, 9627–9640
Kanchanapoom, M.L., Thomas, J.F. (1987) Stereological study of ultrastructural changes in the shoot apical meristem ofNicotiana tabacum during floral induction. Am. J. Bot.74, 152–163
Kofer, W., Glimelius, K., Bonnett, H.T. (1991) Modifications of mitochondrial DNA cause changes in floral development in homeotic-like mutants of tobacco. Plant Cell3, 759–769
Koßmann, J., Müller-Röber, B., Dyer, T.A., Raines, C.A., Sonnewald, U., Willmitzer, L. (1992) Cloning and expression analysis of the plastidic fructose-1,6-bisphosphatase coding sequence from potato: circumstantial evidence for the import of hexoses into chloroplasts. Planta188, 7–12
Krömer, S., Heldt, H.W. (1991) On the role of mitochondrial oxidative phosphorylation in photosynthesis metabolism as studied by the effect of oligomycin on photosynthesis in protoplasts and leaves of barley (Hordeum vulgare). Plant Physiol.95, 1270–1276
Liao, X., Butow, R.A. (1993) BTG1 and RTG2: Two yeast genes required for a novel path of communication form mitochondria to the nucleus. Cell71, 61–71
Logemann, J., Schell, J., Willmitzer, L. (1987) Improved method for the isolation of RNA from plant tissues. Anal. Biochem.163, 21–26
Monéger, F., Mandaron, P., Niogret, M.-F., Freyssinet, G., Mache, R. (1992) Expression of chloroplast and mitochondrial genes during microsporogenesis in maize. Plant Physiol.99, 396–400
Moore, A.L., Beechey, R.B. (1987) Plant mitochondria: Structural, functional and physiological aspects. Plenum Press, New York
Needleman, S.D., Wunsch, C.D. (1970) A general method applicable to the search for similarities in the amino acid sequence of two proteins. J. Mol. Biol.48, 443–453
Ner, S.S., Bhayana, V., Bell, A.W., Giles, I.G., Duckworth, H.W., Bloxham, D.P. (1983) Complete sequence of thegtlA. gene encoding citrate synthase inEscherichia coli. Biochemistry22, 5243–5249
Pearson, W.R., Lipman, D.J. (1988) Improved tools for biological sequence comparison, Proc. Natl. Acad. Sci. USA85, 2444–2448
Pfanner, N., Neupert, W. (1990) The mitochondrial protein import apparatus. Annu. Rev. Biochem.59, 331–353
Raghavendra, A.S., Padmasree, K., Saradadevi, K. (1994) Interdependence of photosynthesis and respiration in plant cells: interactions between chlorSoplasts and mitochondria. Plant Sci.97, 1–14
Remington, S., Weigand, G., Huber, R. (1982) Crystallographic refinement and atomic models of two different forms of citrate synthase at 2.7 Å and 1.7 Å resolution. J. Mol. Biol.158, 111–152
Rios, R., de Buyser, J., Henry, Y., Ambard-Bretteville, F., Rémy, R. (1991) Two-dimensional electrophoretic comparison of mitochondrial polypeptides from different wheat (Triticum aestivum L.) tissues. Plant Sci.76, 159–166
Sambrook, J., Fritsch, E.F., Maniatis, T. (1989) Molecular cloning: A laboratory manual, 2nd edition. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
Sanger, F., Nicklen, S., Coulson, A.R. (1977) DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA74, 5463–5467
Sowokinos, J. (1990a) Effect of stress and senescence on carbon partitioning in stored potatoes. Am. Potato J.67, 849–857
Sowokinos, J. (1990b) Stress-induced alterations in carbohydrate metabolism. In: Molecular and cellular biology of the potato, pp. 137–158, Vayda, M.E., Park, W., eds. C.A.B. International, Wallingford, UK
Spector, L.B. (1972) Citrate cleavage and related enzymes. In: The enzymes, vol. 7, 3rd edn. pp. 357–389, Boyer, P.D., ed. Academic Press, New York and London
Srere, P.A. (1969) Citrate synthase. Methods Enzymol13, 3–11
Suissa, M., Suda, K., Schatz, G. (1984) Isolation of the nuclear yeast gene for citrate synthase and fifteen other mitochondrial proteins by a new screening method. EMBO J.8, 1773–1781
Tobin, A.K., Rogers, W.J. (1992) Metabolic interactions of organelles during leaf development. In: Plant organelles. Compartmentation of metabolism in photosynthetic tissue (Society for experimental biology seminar series 50), pp. 293–323, Tobin, A.K., ed. Cambridge University Press, Cambridge
Turgeon, R. (1989) The sink-source transition in leaves. Annu. Rev. Plant Physiol. Plant Mol. Biol.40, 119–138
Unger, E.A., Hand, J.M., Cashmore, A.R., Vasconcelos, A.C. (1989) Isolation of a cDNA encoding mitochondrial citrate synthase fromArabidopsis thaliana. Plant Mol. Biol.13, 411–418
Van der Krol, A., Mol, J., Stuitje, A. (1988) Modulation of eucaryotic gene expression by complementary RNA or DNA sequences. BioTechniques6, 958–976
Warmke, H.E., Lee, S.-L.J. (1978) Pollen abortion in T cytoplasmic male-sterile corn (Zea mays): A suggested mechanism. Science200, 561–563
Wiskich, J.T., Meidan E. (1992) Metabolic interactions between organelles in photosynthetic tissue: a mitochondrial overview. In: Plant organelles. Compartmentation of metabolism in photosynthetic tissue (Society for experimental biology seminar series 50), pp. 1–19, Tobin, A.K., ed. Cambridge University Press, Cambridge
Author information
Authors and Affiliations
Additional information
The nucleotide sequence data reported will appear in the EMBL, GenBank and DDBJ Nucleotide Sequence Databases under the accession number X75082.
We would like to thank G. Nast for technical assistance. We also thank our gardeners B. Burose and R. Breitfeld for taking care of greenhouse plants, and A. Voigt for photographic work.
Rights and permissions
About this article
Cite this article
Landschütze, V., Müller-Röber, B. & Willmitzer, L. Mitochondrial citrate synthase from potato: predominant expression in mature leaves and young flower buds. Planta 196, 756–764 (1995). https://doi.org/10.1007/BF01106771
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01106771