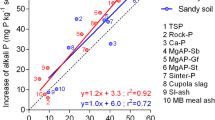
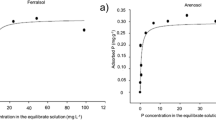
Abstract
Soils overfertilized with phosphorus (P) are widespread in the European Union and there is consensus among soil scientists to better explore their potential to release phosphate. In this work we report the principal physical and chemical properties of twelve overfertilized benchmark soils of contrasting agricultural areas in Italy, Germany, Great Britain, and Spain. The criterion used to consider them as overfertilized was that the available P amount, measured by the regional soil P test, was at least twice as large as the accepted critical level for an average crop. The soils could be usefully divided into three groups, calcareous, slightly acidic and acidic based upon their basic chemical properties and reactions and proportion of the major P fractions (NaOH- plus citrate-bicarbonate-, citrate-bicarbonate-dithionite-, and HCl-extractable P). Six extraction procedures commonly used to evaluate potentially plant-available P (Olsen, anion-exchange resin (resin-), anion plus cation-exchange resin (resin±), Ca acetatelactate (CAL), water, and Fe oxide-impregnated paper strips (strip) were compared. The extractable P values by each method were correlated but the amount of P extracted varied and differed in the order water-P< Olsen-P< CAL-P< strip-P< resin(±)-P<resin(−)-P. Olsen-P and strip-P extracted a proportion of the more available P fraction (NaOH- and citrate-bicarbonate-extractable P), which increased with increasing pH, and decreasing amounts of active Fe and Al forms in soil. Consequently, pH can be used in conjunction with simple soil P tests to provide a first evaluation of potentially releaseable P.
Similar content being viewed by others
References
Barrow NJ (1983) A mechanistic model for describing sorption and desorption of phosphate by soil. J Soil Sci 34:73–750
Castro B & Torrent J (1995) Phosphate availability in calcareous Vertisols and Inceptisols in relation to fertilizer type and soil proprerties. Fert Res 40:109–119
Chung FH (1973) Quantitative interpretation of X-ray diffraction patterns of mixtures. J Appl Cryst 7:519–525
Drouineau G (1942) Dosage rapide du calcaire actif du sol: nouvelles données sur la separation et la nature des fractions calcaires. Ann Agron 12:441–450
FAO-Unesco (1988) Soil Map of the World. Revised Legend. Amended Final Draft. FAO, Rome, Italy.
Freese D, van der Zee SEATM & van Riemsdijk WH (1992) Comparison of different models for phosphate sorption as a function of the iron and aluminium oxides of soils. J Soil Sci 43:729–738
Gerke J (1993) Solubilization of Fe(III) from humic complexes, humic/Fe-oxide mixtures and from poorly ordered Fe-oxide by organic acids - consequences for P adsorption. Z Pflanzenernähr Bodenkd 156:253–257
Gerke J & Hermann R (1992) Adsorption of orthophosphate to humic-Fe-complexes and to amorphous Fe-oxide. Z Pflanzenähr Bodenkd 155:233–236
House WA & Casey H (1989) Transport of phosphorus in rivers.In Tiessen H (ed) Phosphorus cycles in Terrestrial and Aquatic Ecosystems, pp 253-282. Proceedings of the SCOPE and UNEP 7: Europe. May 1 - May 6, 1988, Cerniejewo, Poland
Kumar V, Bolland MDA & Gilkes RJ (1994) Comparison of the Pi, Colwell, Bray1, calcium acetate lactate (CAL) and Truog soil phosphorus test for predicting growth of oats, barley, triticale and clover in the field in lateritic soils fertilised with superphosphate and rock phosphate. Fert Res 37:115–124
Mehra OP & Jackson ML (1960) Iron oxide removal from soils and clays by a dithionite-citrate system buffered with sodium bicarbonate. Clays Clay Miner 7:317–327
Murphy J & Riley JP (1962) A modified single solution method for determination of phosphate in natural waters. Anal Chim Acta 27:31–36
Olsen SR, Cole CV, Watanabe FS & Dean LA (1954) Estimation of available phosphorus in soils by extraction with sodium bicarbonate. USDA Circ 939. US Gov Print Office, Washington, USA
Olsen SR & Sommers LE (1982) Phosphorus.In: Page AL, Miller RH & Keeney DR (eds) Methods of Soil Analysis. Part 2, 2nd ed. Agronomy 9 pp 403–430 ASA, SSSA, Madison, WI, USA
Pennell KD, Boyd SA & Abriola LM (1995) Surface area of soil organic matter reexamined. Soil Sci Soc Am J 59:1012–1018
Pierzynski GM & Logan TJ (1993) Crop, soil and management effects on phsphorus soil test levels. J Prod Agric 6:513–520
Pierzynski GM, Logan. TJ, Traina SJ & Bigham JM (1990) Phosphorus chemistry and mineralogy of excessively fertilized soils: Description of phosphorus-rich particles. Soil Sci Soc Am J 54:1583–1589
Reith JWS, Inkson RHE, Scott NM, Caldwell KS, Ross JAM & Simpson WE (1987) Estimates of soil phosphorus for different soil series. Fert Res 11:123–143
Sanyal SK & De Datta SK (1991) Chemistry of phosphorus transformations in soil. Adv Soil Sci 16:1–120
Schüller M (1969) Die CAL Methode, eine neue methode zur Bestimmung des pflanzenverfuegbaren Phosphates in Böden. Z. Pflanzenernaehr Bodenkd 123:48–63
Schulze DG (1981) Identification of soil iron oxide minerals by differential X-ray diffraction. Soil Sci Soc Am J 45:437–440
Schwertmann U (1964) Differenzierung der Eisenoxide des Bodens durch Extraktion mit Ammoniumoxalat-Lösung. Z Pfanzenernähr Düng Bodenkd 105:194–202
Sharpley AN (1995) Dependence of runoff phosphorus on extractable soil phosphorus. J Environ Qual 24:920–926
Sharpley AN & Ahuja LR (1983) A diffusion intepretation of soil phosphorus desorption. Soil Sci 135:322–326
Sharpley AN, Hedley MJ, Sibbesen E, Hillbricht-Ilkowska A, House WA & Ryszkaoski L (1995) Phosphorus transfer from terrestrial to aquatic ecoystems.In: Tiessen H (ed) Phosphorus in the global Environment. Transfer Cycles and Management. SCOPE 54, pp 171–199 J Wiley and Sons, New York, USA
Sharpley AN, Ahuja LR, Yamamoto M & Menzel RG (1981) The kinetics of phosphorus desorption from soil. Soil Sci Soc Am J 45:493–496
Sharpley AN & Withers PJA (1994) The envirormentally-sound management of agricultural phosphorus. Fert Res 39:133–146
Sibbesen E (1978) An investigation of the anion-exchange resin method for soil phosphate extraction. Plant Soil 50:305–321
Sibbesen E & Runge-Metzer A (1995) Phosphorus balance in European Agriculture - Status and Policy OptionsIn: Tiessen H (ed) Phosphorus in the global Environment. Transfer, Cycles and Management. SCOPE. 54, pp 43–57 J. Wiley and Sons, New York, USA
Somasiri LLW & Edwards AC (1992) An ion-exchange resin method for nutrient extraction of agricultural advisory soil samples. Commun Soil Sci Plant Anal 23:645–667
Van der Zee SEATM, Fokkink LGJ & van Riemsdijk WH (1987) A new technique for assessment of reversibly adsorbed phosphate. Soil Sci Soc Am J 51:599–604
Van Wesemael JCh (1955) De bepaling van het calciumcarbonaatgehalte van gronden. Chem Weekblad 51:35–36
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Barberis, E., Ajmone Marsan, F., Scalenghe, R. et al. European soils overfertilized with phosphorus: Part 1. Basic properties. Fertilizer Research 45, 199–207 (1995). https://doi.org/10.1007/BF00748590
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00748590