Summary
The receptive fields of receptor cells and one class of first order interneurons have been investigated using several methods.
-
1.
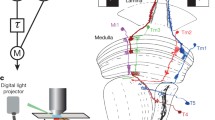
The spatial modulation transfer function (MTF) has been measured intracellularly from photoreceptors (R1–6) and one class of first order interneurons (LMC's) in the fliesMusca domestica, Lucilia sericata andCalliphora stygia (R1–6: Figs. 2–4; LMC's: Figs. 5–7). Moving sine wave gratings of 25 different spatial frequencies were used to stimulate the cells.
-
2.
The angular sensitivity function corresponding to each MTF was computed by taking the inverse Fourier transform. All cells have a higher sensitivity to light from wide angles than is expected of a Gaussian of the same half width (Fig. 4). No significant changes in the angular sensitivity function of receptor cells are observed when the mean light intensity and the temporal frequency of the stimulus grating is varied within the limited range available.
-
3.
By comparison, the LMC's change their MTF significantly when the mean light intensity and the temporal frequency of the grating are altered (Figs. 6 and 7). The computed angular sensitivity function exhibits inhibitory flanks at high mean intensity and low temporal frequency, which disappear at low mean intensity and high temporal frequency.
-
4.
The receptive fields of receptors and LMC's were also tested using single bars of different widths. The response to sinusoidal modulation of the bar's intensity was measured as a function of its width (Figs 9–12). The results from receptors confirm the fields obtained from the MTF's (Figs. 9 and 10). The responses of second order neurons were more distorted and decreased in amplitude when the width of the bar exceeded the purely excitatory field (approx. 3 degrees inLucilia. cuprina) (Figs. 11 and 12). With this technique, only small differences could be detected between inhibitory effects at different mean light intensities. — The transfer function of the first synapse, while adding some distortions to the waveform, also rectifies some of the distortions due to the receptor MTF, namely the modulation asymmetry with respect to the response to the mean intensity (Figs. 10, 12 and 13).
-
5.
The impulse response functions of photoreceptors to a light source positioned on-axis and off-axis were measured (Figs. 14 and 16). A much slower response was obtained when the light source was stimulating mainly the neighbouring receptors, suggesting coupling between receptors of different optical axis. Identical experiments were also carried out in the LMC's (Figs. 15 and 18). In the LMC's at very low stimulus intensity, the receptor coupling is reflected in a similar slower response as observed in the receptors. With increasing stimulus intensity, lateral inhibition reduces the response to off-axis light (Fig. 15).
-
6.
Three point sources were used to determine whether inhibition in the fly lamina is recurrent. It was shown that while one spot of light can inhibit the response invoked by another, a third spot of light does not decrease this inhibitory effect (Figs. 19 and 20) suggesting that inhibition is not recurrent.
Similar content being viewed by others
Abbreviations
- ERG :
-
electroretinogram
- LMC :
-
lamina monopolar cell
- LED :
-
light emitting diode
- MTF :
-
modulation transfer function
- Δp :
-
width at half height of the angular sensitivity function
References
Arnett DW (1972) Spatial and temporal integration properties of units in the first optic ganglion of dipterans. J Neurophysiol 35:429–444
Ashmore JF, Falk G (1980a) Responses of rod bipolar cells in the dark-adapted retina of the dogfish,Scyliorhinus canicula. J Physiol (Lond) 300:115–150
Ashmore JF, Falk G (1980b) The single-photon signal in rod bipolar cells of the dogfish retina. J Physiol (Lond) 300:151–166
Autrum H, Zettler F, Järvilehto M (1970) Postsynaptic potentials from a single monopolar neuron of the ganglion opticum I of the blowflyCalliphora. Z Vergl Physiol 70:414–424
Barlow HB (1961) Possible principles underlying the transformations of sensory messages. In: Rosenblith WA (ed) Sensory communication. MIT Press, Cambridge, MA, pp 217–234
Barlow HB (1972) Dark and light adaptation: psychophysics. In: Jameson D, Hurvich LM (ed) Handbook of sensory physiology, vol VII/4. Springer, Berlin Heidelberg New York
Barlow HB, Levick WR (1976) Threshold setting by the surround of cat retinal ganglion cells. J Physiol (Lond) 259:737–757
Barlow HB, Fitzhugh R, Kuffler SW (1957) Change of organization in the receptive fields of the cat's retina during dark adaptation. J Physiol (Lond) 137:338–354
Beersma D (1979) Spatial characteristics of the visual field of the flies. PhD thesis. Rijksuniversiteit te Groningen
Brodie SE, Knight BW, Ratliff F (1978a) The response of theLimulus retina to moving stimuli: a prediction by Fourier synthesis. J Gen Physiol 72:129–165
Brodie SE, Knight BW, Ratliff F (1978b) The spatiotemporal transfer function of theLimulus lateral eye. J Gen Physiol 72:167–202
Chi C, Carlson SD, St. Marie RL (1979) Membrane specializations in the peripheral retina of the houseflyMusca domestica L. Cell Tissue Res 198:501–520
Daitch JM, Green DG (1969) Contrast sensitivity of the human peripheral retina. Vision Res 9:947–952
Dubs A (1981) Non-linearity and light adaptation in the fly photoreceptor. J Comp Physiol 144:53–59
Dubs A, Laughlin SB, Srinivasan MV (1981) Single photon signals in the fly photoreceptors and first interneurons at behavioural threshold. J Physiol (Lond) 317:317–334
Dvorak DR, Snyder AW (1978) The relationship between visual acuity and illumination in the fly,Lucilia sericata. Z Naturforsch 33c:139–143
Dvorak DR, Srinivasan MV, French AS (1980) The contrast sensitivity of fly movement-detecting neurons. Vision Res 20:397–407
Enroth-Cugell C, Robson JG (1966) The contrast sensitivity of retinal ganglion cells of the cat. J Physiol (Lond) 187:517–552
Franceschini N, Hardie RC, Ribi W, Kirschfeld K (1981) Sexual dimorphism in a photoreceptor. Nature 291:241–244
Götz KG (1964) Optomotorische Untersuchung des visuellen Systems einiger Augenmutanten der FruchtfliegeDrosophila. Kybernetik 2:77–92
Götz KG (1965) Die optischen Übertragungseigenschaften der Komplexaugen vonDrosophila. Kybernetik 2:215–221
Hardie RC (1979) Electrophysiological analysis of fly retina. I: Comparative properties of R1–6 and R7 and R8. J Comp Physiol 129:19–33
Hartline HK (1968) Visual receptors and retinal interaction. In: Les Prix Nobel en 1967. The Nobel Foundation, Stockholm, pp 242–269
Hartline HK, Ratliff F (1957) Inhibitory interaction of receptor units in the eye ofLimulus. J Gen Physiol 40:357–376
Horridge GA, Mimura K, Hardie RC (1976) Fly photoreceptors III. Angular sensitivity as a function of wavelength and the limits of resolution. Proc R Soc Lond [Biol] 194:151–177
Järvilehto M, Zettler F (1971) Localized intracellular potentials from pre- and post-synaptic components in the external plexiform layer of an insect retina. Z Vergl Physiol 75:422–440
Kaneko A (1973) Receptive field organization of bipolar and amacrine cells in the goldfish retina. J Physiol (Lond) 235:133–153
Kirschfeld K (1967) Die Projektion der optischen Umwelt auf das Raster der Rhabdomere im Komplexauge vonMusca. Exp Brain Res 3:248–270
Kuffler SW (1953) Discharge patterns and functional organization of the mammalian retina. J Neurophysiol 16:37–68
Laughlin SB (1973) Neural integration in the first optic neuropile of dragonflies. I. Signal amplification in dark adapted second order neurons. J Comp Physiol 84:335–355
Laughlin SB (1974a) Neural integration in the first optic neuropile of dragonflies. II. Receptor signal interactions in the lamina. J Comp Physiol 92:357–375
Laughlin SB (1974b) Neural integration in the first optic neuropile of dragonflies. III. The transfer of angular information. J Comp Physiol 92:377–396
Laughlin SB (1976) Adaptations of the dragonfly retina for contrast detection and the elucidation of neural principles in the peripheral visual system. In: Zettler F, Weiler R (eds) Neural principles in vision. Springer, Berlin Heidelberg New York, pp 175–193
Laughlin SB (in press) A simple coding procedure enhances a neuron's information capacity. Z Naturforsch 36c
Laughlin SB, Hardie RC (1978) Common strategies for light adaptation in the peripheral visual systems of fly and dragonfly. J Comp Physiol 128:319–340
Leutscher-Hazelhoff JT (1975) Linear and non-linear performance of transducer and pupil inCalliphora retinula cells. J Physiol (Lond) 246:333–350
Menzel R, Blakers M (1976) Colour receptors in the bee eye — morphology and spectral sensitivity. J Comp Physiol 108:11–33
Mimura K (1976) Some spatial properties in the first ganglion of the fly. J Comp Physiol 105:65–82
Mimura K (1978) Electrophysiological evidence for interaction between retinula cells in the flesh-fly. J Comp Physiol 125:209–216
Mimura K (1981) Receptive field patterns in photoreceptors of the fly. J Comp Physiol 141:349–362
Muijser H (1979) The receptor potential of retinular cells of the blowflyCalliphora: the role of sodium, potassium and calcium ions. J Comp Physiol 132:87–95
Pick B (1977) Specific misalignments of rhabdomere visual axes in the neural superposition eye of dipteran flies. Biol Cybern 26:215–224
Pick B, Buchner E (1979) Visual movement detection under light- and dark-adaptation in the fly,Musca domestica. J Comp Physiol 134:45–54
Pirenne MH (1946) On the variation of visual acuity with light intensity. Proc Cambridge Philos Soc 42:78–82
Pirenne MH (1967) Vision and the eye, 2nd ed. Chapman and Hall, London, pp 141–152
Rall W (1969) Time constants and electronic length of membrane cylinders and neurons. Biophys J 9:1483–1508
Ribi WA (1978) Gap junctions coupling photoreceptor axons in the first optic ganglion of the fly. Cell Tissue Res 195:299–308
Schwartz EA (1974) Responses of bipolar cells in the retina of the turtle. J Physiol (Lond) 236:211–224
Schwartz EA (1975a) Rod-rod interaction in the retina of the turtle. J Physiol (Lond) 246:617–638
Schwartz EA (1975b) Cones excite rods in the retina of the turtle. J Physiol (Lond) 246:639–651
Shaw SR (1967) Simultaneous recording from two cells in the locust retina. Z Vergl Physiol 55:183–194
Shaw SR (1968) Organization of the locust retina. Symp Zool Soc (Lond) 23:135–163
Shaw SR (1969a) Interreceptor coupling in ommatidia of the drone honey-bee and locust compound eye. Vision Res 9:999–1029
Shaw SR (1969b) Sense-cell structure and interspecies comparisons of polarized-light absorption in arthropod compound eyes. Vision Res 9:1031–1040
Shaw SR (1975) Retinal resistance barriers and electrical lateral inhibition. Nature 255:480–483
Shaw SR (1978) The extracellular space and blood-eye barrier in an insect retina: An ultrastructural study. Cell Tissue Res 188:35–61
Shaw SR (1981) Anatomy and physiology of identified non-spiking cells in the photoreceptor-lamina complex of the compound eye of insects, especially Diptera. In: Roberts A, Bush BMH (eds) Neurones without impulses. Society for Experimental Biology. Seminar Series 6. Cambridge University Press, Cambridge
Srinivasan MV, Dvorak DR (1980) Spatial processing of visual information in the movement-detecting pathway of the fly. J Comp Physiol 140:1–23
Strausfeld NJ, Campos-Ortega JA (1977) Vision in insects: pathways possibly underlying neural adaptation and lateral inhibition. Science 195:894–897
Streck P (1972) Der Einfluß des Schirmpigmentes auf das Sehfeld einzelner Sehzellen der FliegeCalliphora erythrocephala Meig. Z Vergl Physiol 76:372–402
Trujillo-Cenóz O (1972) The structural organisation of the compound eye in insects. In: Fuortes MGF (ed) Handbook of sensory physiology, vol VII/2. Springer, Berlin Heidelberg New York, pp 5–62
Tunstall J, Horridge GA (1967) Electrophysiological investigation of the optics of the locust retina. Z Vergl Physiol 55:167–182
Werblin FS (1978) Transmission along and between rods in the tiger salamander retina. J Physiol (Lond) 280:449–470
Werblin F, Dowling JE (1969) Organisation of the retina of the mudpuppy,Necturus maculosus. II. Intracellular recording. J Neurophysiol 32:339–355
Zettler F, Autrum H (1975) Chromatic properties of lateral inhibition in the eye of a fly. J Comp Physiol 97:181–188
Zeltler F, Järvilehto M (1970) Histologische Lokalisation der Ableitelektrode. Belichtungspotentiale aus Retina und Lamina beiCalliphora. Z Vergl Physiol 68:202–210
Zeltler F, Järvilehto M (1972) Lateral inhibition in an insect eye. Z Vergl Physiol 76:233–244
Zettler F, Weiler R (1976) Neural processing in the first optic neuropile of the compound eye of the fly. In: Zettler F, Weiler R (eds) Neural principles in vision. Springer, Berlin Heidelberg New York, pp 227–237
Zimmerman RP (1978) Field potential analysis and the physiology of second-order neurons in the visual system of the fly. J Comp Physiol 126:297–316
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Dubs, A. The spatial integration of signals in the retina and lamina of the fly compound eye under different conditions of luminance. J. Comp. Physiol. 146, 321–343 (1982). https://doi.org/10.1007/BF00612703
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00612703