Abstract
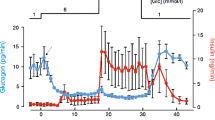
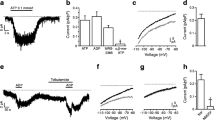
K+ currents through ATP-dependent channels were recorded from inside-out patches of β-cell membrane as previously described (Rorsman and Trube 1985). Channels were opened by removing ATP from the intracellular side of the membrane. The open probability and/or the number of active channels declined spontaneously (“run-down”) when ATP was absent for periods longer than about 30 s. Channels subject to the run-down could be activated again after applying a blocking concentration (>0.1 mM) of ATP in presence of 1 mM MgCl2 for at least 2 min. ATP in absence of Mg and the ATP-analogues AMP-PNP, AMP-PCP and ATPγS were ineffective in reactivating the channels. This suggests that phosphorylation of the channels or associated proteins of hydrolysis of ATP may be necessary for keeping the channels available. In contrast to the differential effects on the run-down, ATP in presence and absence of Mg and the ATP analogues were similarly effective in blocking the channels at concentrations above 0.1 mM. Using an experimental protocol avoiding the run-down ghe dose-inhibition curve for ATP was found to reach 50% at 18 μM.
Similar content being viewed by others
References
Ashcroft FM, Harrison DE, Ashcroft SJH (1984) Glucose induces closure of single potassium channels in isolated rat pancreatic β-cells. Nature 312:446–448
Byerly L, Hagiwara S (1982) Calcium currents in internally perfused nerve cell bodies ofLymnaea stagnalis. J Physiol (Lond) 322:503–528
Byerly L, Yazejian B (1986) Intracellular factors for the maintenance of calcium currents in perfused neurones from the snail,Lymnaea stagnalis. J Physiol (Lond) 370:631–650
Chad JE, Eckert R (1986) An enzymatic mechanism for calcium current inactivation in dialyzedHelix neurones. J Physiol (Lond) 378:31–51
Cook DL, Hales N (1984) Intracellular ATP directly blocks K+ channels in pancreatic β-cells. Nature 311:271–273
Cook DL, Ikeuchi M, Fujimoto WY (1984) Lowering of pHi inhibits Ca2+-activated K+ channels in pancreatic β-cells. Nature 311:269–271
Doroshenko PA, Kostyuk PG, Martynyuk AI (1982) Intracellular metabolism of adenosine-3′,5′-cyclic monophosphate and calcium inward current in perfused neurones ofHelix pomatia. Neuroscience 7:2125–2134
Doroshenko PA, Kostyuk PG, Martynyuk AE, Kursky MD, Vorobetz ZD (1984) Intracellular protein kinase and calcium inward currents in perfused neurones of the snailHelix pomatia. Neuroscience 11:263–267
Fenwick EM, Marty A, Neher E (1982a) A patch-clamp study of bovine chromaffin cells and of their sensitivity to acetylcholine. J Physiol (Lond) 331:577–597
Fenwick EM, Marty A, Neher E (1982b) Sodium and calcium channels in bovine chromaffin cells. J Physiol (Lond) 331:599–635
Findlay I, Dunne MJ (1986) ATP maintains ATP-inhibited K+ channels in an operational state. Pflügers Arch 407:238–240
Findlay I, Dunne MJ, Petersen OH (1985a) High-conductance K+ channel in pancreatic islet cells can be activated and inactivated by internal calcium. J Membrane Biol 83:169–175
Findlay I, Dunne MJ, Ullrich S, Wollheim CB, Petersen OH (1985b) Quinine inhibits Ca2+-independent K+ channels whereas tetraethylammonium inhibits Ca2+-activated K+ channels in insulin secreting cells. FEBS Lett 185:4–8
Findlay I, Dunne MJ, Petersen OH (1985c) ATP-sensitive inward rectifier and voltage- and calcium-activated K+ channels in cultured pancreatic islet cells. J Membr Biol 8:165–172
Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ (1981) Improved patch-clamp techniques for high resolution current recording from cells and cell-free membrane patches. Pflügers Arch 391:85–100
Hedeskov CJ (1980) Mechanisms of glucose-induced insulin secretion. Physiol Rev 60:442–509
Kakei M, Noma A, Shibasaki T (1985) Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol (Lond) 363:441–462
Lernmark A (1974) The preparation of, and studies on, free cell suspensions from mouse pancreatic islets. Diabetologia 10:431–438
Martell AE, Smith RM (1974) Critical stability constants, vol 1, Amino acids; vol 2, Amines. Plenum Press, New York
Misler S, Falke LC, Gillis K, McDaniel ML (1986) A metaboliteregulated potassium channel in rat pancreatic B cells. Proc Natl Acad Sci USA 83:7119–7123
Noma A (1983) ATP-regulated K+ channels in cardiac muscle. Nature 305:147–148
Palvimo J, Linnala-Kankkunen A, Mäenpää PH (1985) Thiophosphorylation and phosphorylation of chromatin proteins from calf thymus in vitro. Biochem Biophys Res Commun 126:103–108
Rorsman P, Trube G (1985) Glucose dependent K+-channels in pancreatic β-cells are regulated by intracellular ATP. Pflügers Arch 405:305–309
Sakmann B, Neher E (1983) Geometric parameters of pipettes and membrane patches. In: Sakmann B, Neher E (eds) Single channel recording, cht 2. Plenum Press, New York, pp 37–51
Spruce AE, Standen NB, Stanfield PR (1985) Voltage-dependent ATP-sensitive potassium channels of skeletal muscle membrane. Nature 316:736–738
Trube G, Hescheler J (1984) Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflügers Arch 401:178–184
Yount RG (1975) ATP analogs. Adv Enzymol 43:1–56
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ohno-Shosaku, T., Zünkler, B.J. & Trube, G. Dual effects of ATP on K+ currents of mouse pancreatic β-cells. Pflugers Arch. 408, 133–138 (1987). https://doi.org/10.1007/BF00581342
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00581342