Abstract
Tobacco mosaic virus protein in phosphate buffer pH 6.5–7.0 (I=0.1 M) shows endothermic polymerization accompanied by water release of the capsomers. At protein concentrations c ∼ 2 mg/ml the transition temperature is T *=20 ± 1‡ C. As indicated by the increase of the partial specific folume (δV 2=0.0049 ± 0.0003 cm3/g) in going from A-protein to helical rods at pH 6.50, the assembly reaction is expected to be inhibited by high pressure; the corresponding isobars of the endothermic polymerization should be shifted to higher T * values.
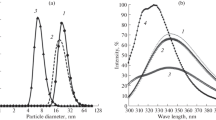
Turbidity measurements at pressures 1<p<1,500 bar are in agreement with the given hypothesis: both, double discs and helical rods are found to be dissociated at elevated pressure, the latter showing somewhat higher stability. At 700 bar the transition temperature of helix formation is shifted by 14‡ C to higher temperatures.
Complete reversibility of the pressure dependent dissociation-association without “hysteresis” proves the process to represent a true equilibrium. At low temperatures and high pressures the association equilibrium is shifted to a molecular weight distribution with M w< M (A-protein). Increased co operativity in the transition A-protein → helical rods, as well as an apparent inversion of the sign of the reaction volume at high temperatures and pressures are caused by pressure induced pH shifts. Adjusting the pH at high pressure to the value at ambient pressure allows to eliminate both effects.
The product of association at high pressure differs in its conformation from the end product obtained from the endothermic polymerization at 1 bar and subsequent pressure application.
Similar content being viewed by others
References
Lauffer MA (1975) Entropy driven processes in biology. Springer, Berlin Heidelberg New York
Kauzmann W (1959) Some factors in the interpretation of protein denaturation. Adv Protein Chem 14: 1–63
Lauffer MA, Ansevin AT, Cartwright TE, Brinton CC Jr (1958) Polymerization-depolymerization of TMP-protein. Nature 181: 1338–1339
Jaenicke R, Lauffer MA (1969) Polymerization-depolymerization of TMV-protein. XII. Further studies on the role of water. Biochemistry 8: 3083–3092
Sturtevant JM, Velicelebi G, Jaenicke R, Lauffer MA (1981) A scanning calorimetric investigation of the polymerization of the coat protein of TMV. Biochemistry (in press)
Lauffer MA, Dow RB (1941) The denaturation of TMV at high pressure. J Biol Chem 140: 509–518
Durham ACH, Finch JT, Klug A (1971) States of aggregation of TMV protein. Nature [New Biol] 229: 37–42
Durham ACH, Klug A (1971) Polymerization of TMV protein and its control. Nature [New Biol] 229: 42–46
Butler PJG, Durham ACH (1977) TMV protein aggregation and the virus assembly. Adv Protein Chem 31: 188–247
Boedtker H, Simmons NS (1958) The preparation and characterization of essentially uniform TMV particles. J Am Chem Soc 80: 2550–2556
Leberman R (1966) The isolation of plant viruses by means of “simple” coacervates. Virology 30: 341–347
Fraenkel-Conrat H (1957) Degradation of TMV with acetic acid. Virology 4: 1–4
Smith CE, Lauffer MA (1967) Polymerization-depolymerization of TMV protein. VIII. Light scattering studies. Biochemistry 6: 2457–2465
Lüdemann H-D, Mahon WAS (1969) Absorption spectra at high temperatures and pressures IV: A new optical high pressure cell for corrosive liquids. High Temp High Pressures 1: 215–220
Schade BC, Lüdemann H-D, Jaenicke R (1980) Reversible high-pressure dissociation of lactic dehydrogenase from pig muscle. Biochemistry 19: 1121–1126
Vogel D, de Marcillac GD, Hirth L, Gregori E, Jaenicke R (1979) Size distribution in the higher stages of polymerization of the A-protein of TMV (vulgare). Z Naturforsch 34c: 782–792
Schuster TM, Scheele RB, Khairallah LH (1979) Mechanism of self-assembly of TMV protein: I. Nucleation-controlled kinetics of polymerization. J Mol Biol 127: 461–485
Shire SJ, Steckert JJ, Schuster TM (1979) Mechanism of self-assembly of TMV protein: II. Characterization of the metastable polymerization nucleus and the initial stages of helix formation. J Mol Biol 127: 487–506
Neuman RC Jr, Kauzmann W, Zipp A (1973) Pressure dependence of weak acid ionization in aqueous buffers. J Phys Chem 77: 2687–2691
Srinivasan S, Lauffer MA (1973) Polymerization studies on protein from the Dahlemense Strain of TMV: Light scattering and related studies. Arch Biochem Biophys 158: 53–66
Jaenicke R (1981) Enzymes under extremes of physical conditions. Annu Rev Biophys Bioeng 10 (in press)
Jaenicke R (1971) Volume changes in the isoelectric heat aggregation of serum albumin. Eur J Biochem 21: 110–115
Durham ACH, Vogel D, de Marcillac GD (1977) Hydrogen-ion binding by TMV protein polymers. Eur J Biochem 79: 151–159
Vogel D, Durham ACH, de Marcillac GD (1977) Metastable aggregates in the polymerization of TMV protein. Eur J Biochem 79: 161–171
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Jaenicke, R., Lüdemann, HD. & Schade, B.C. High pressure effects on the endothermic association of tobacco mosaic virus protein. Biophys. Struct. Mechanism 7, 195–203 (1981). https://doi.org/10.1007/BF00539179
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00539179