Summary
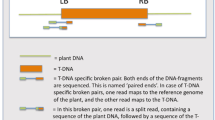
The structure of the T-DNA in Ri-transformed plants of Brassica napus, Nicotiana plumbaginifolia and Nicotiana tabacum was analysed. All the plants studied present a particular phenotype with wrinkled leaves. The T-DNA is composed of two parts: TL and TR. The size of the TL-DNA (19–20 kb) seems to be almost constant, except in N. tabacum where it is shorter. The TR-DNA can be absent, and its size varies from about 5–28 kb, with two predominant lengths. The smaller size does not include the region homologous to the tms genes of the pTi T-DNA. The copy number varies from one to four copies per plant genome. TL and TR-DNA are not always present in the same copy number, but in some cases are linked together.
Similar content being viewed by others
References
Ackermann C (1977) Planzen aus Agrobacterium rhizogenes Tumoren aus Nicotiana tabacum. Plant Sci Lett 8:23–30
Barker RF, Idler KB, Thompson DV, Kemp JD (1983) Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plasmid pTi15855. Plant Mol Biol 2:335–350
Birnboin MC, Doly J (1979) A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res 7:1513–1523
Bourgin JP (1978) Valine-resistant plants from in vitro selected tobacco cells. Mol Gen Genet 161:225–230
Byrne MC, Koplow J, David C, Tempe J, Chilton MD (1983) Structure of T-DNA in roots transformed by Agrobacterium rhizogenes. J Mol Appl Genet 2:201–209
Chilton MD, Tepfer DA, Petit A, David C, Casse-Delbart F, Tempe J (1982) Agrobacterium rhizogenes inserts T-DNA into the genome of the host plant root cells. Nature 295:432–434
Costantino P, Spano L, Pomponi M, Benvenuto E, Ancora G (1984) The T-DNA of Agrobacterium rhizogenes is transmitted through meiosis to the progeny of hairy root plants. J Mol Appl Genet 2:465–470
David C, Chilton MD, Tempe J (1984) Conservation of T-DNA in plants regenerated from hairy root cultures. Bio/technology 2:73–76
Dellaporta SL, Wood J, Hicks JB (1983) A plant DNA minipreparation: Version II. Plant Mol Biol Reporter 1:19–21
De Paolis A, Mauro ML, Pomponi M, Cardarelli M, Spano L, Costantino P (1985) Localization of agropine-synthesizing functions in the TR region of the root-inducing plasmid of Agrobacterium rhizogenes 1855. Plasmid 13:1–7
Durand-Tardif M, Broglie R, Slightom J, Tepfer D (1985) Structure and expression of Ri T-DNA from Agrobacterium rhizogenes in Nicotiana tabacum. Organ and phenotypic specificity. J Mol Biol 186:557–564
Garfinkel DJ, Simpson RB, Ream LW, White FF, Gordon MP, Nester EW (1981) Genetic analysis of crown-gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell 27:143–153
Gielen J, de Beuckeleer M, Seurinck J, Deboeck F, de Greve H, Lemmers M, van Montagu M, Schell J (1984) Complete nucleotide sequence of the TL-DNA of the Agrobacterium tumefaciens plasmid pTiAch5. EMBO J 3:835–846
Guerche P, Jouanin L, Tepfer D, Pelletier G (1987) Genetic transformation of oilseed rape (Brassica napus) by Ri T-DNA of Agrobacterium rhizogenes and analysis of inheritance of the transformed phenotype. Mol Gen Genet 206:382–386
Huffman GA, White FF, Gordon MP, Nester GW (1984) Hairy root inducing plasmid: Physical map and homology to tumor-inducing plasmids. J Bacteriol 157:269–276
Inze D, Follin A, van Lijsebettens M, Simoens C, Genetello C, van Montagu M, Schell J (1984) Genetic analysis of the individual T-DNA genes of Agrobacterium tumefaciens; further evidence that two genes are involved in indole-3-acetic acid synthesis. Mol Gen Genet 194:265–274
Joos H, Inze D, Caplan A, Sormann M, van Montagu M, Schell J (1983) Genetic analysis of T-DNA transcripts in nopaline crown-galls. Cell 32:1057–1067
Jouanin L (1984) Restriction map of an agropine-type Ri plasmid and its homologies with Ti plasmids. Plasmid 12:91–102
Lahners K, Byrne MC, Chilton MD (1984) T-DNA fragments of hairy root plasmid pRi8196 are distantly related to octopine and nopaline Ti plasmid T-DNA. Plasmid 11:130–140
Leach F (1983) Etude de la région TL du plasmide Ri d'Agrobacterium rhizogenes souche A4. Thèse de troisième cycle, Université de Paris Sud, Centre d'Orsay
Lemmans J, Deblaere R, Willmitzer L, de Greve H, Hernalsteens JP, van Montagu M, Schell J (1982) Genetic identification of functions of TL-DNA transcripts in octopine crown-gall. EMBO J 1:147–152
Maniatis T, Fritsch EF, Sambrook J (1982) Molecular cloning, a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY
Ooms G, Hooykaas PJJ, Mooleman G, Schilperoort RA (1981) Crown gall plant tumors of abnormal morphology, induced by Agrobacterium tumefaciens carrying mutated octopine Ti plasmids; analysis of T-DNA functions. Gene 14:33–50
Peralta EG, Ream LW (1985) T-DNA border sequences required for crown gall tumorigenesis. Proc Natl Acad Sci USA 82:5112–5116
Salomon F, Deblaere R, Leemans J, Hernalsteens JP, van Montagu M, Schell J (1983) Genetic identification of functions of TR-DNA transcripts in octopine crown-galls. EMBO J 3:141–146
Schroder C, Waffenschmidt S, Weiler EW, Schroder J (1984) The T-region of Ti plasmids codes for an enzyme synthesizing indole-3-acetic acid. Eur J Biochem 138:387–391
Shaw CH, Watson MD, Carter GM, Shaw CM (1984) The right hand copy of the nopaline Ti-plasmid 25 bp repeat is required for tumor formation. Nucleic Acids Res 12:6031–6041
Slightom JL, Jouanin L, Leach F, Drong RF, Tepfer D (1985) Isolation and identification of TL-DNA/plant junctions in Convolvulus arvensis transformed by Agrobacterium rhizogenes strain A4. EMBO J 4:3069–3077
Slightom JL, Durand-Tardif M, Jouanin L, Tepfer D (1986)Nucleotide sequence analysis of TL-DNA of Agrobacterium rhizogenes agropine type plasmid: identification of open-reading frames. J Biol Chem 261:108–121
Spano L, Pomponi M, Costantino P, van Slogteren GMS, Tempe J (1982) Identification of T-DNA in the root-inducing plasmid of the agropine type Agrobacterium rhizogenes 1855. Plant Mol Biol 1:291–300
Taylor BH, Amasino RM, White FF, Nester EW, Gordon MP (1985) T-DNA analysis of plants regenerated from hairy root tumors. Mol Gen Genet 201:554–557
Tepfer D (1983) The biology of genetic transformation of higher plants by Agrobacterium rhizogenes. In: Puhler A (ed) Molecular Genetics of the Bacteria-Plant Interaction, Springer, Berlin, Heidelberg, pp 248–258
Tepfer D (1984) Genetic transformation of several species of higher plants by Agrobacterium rhizogenes: phenotypic consequences and sexual transmission of the transformed genotype and phenotype. Cell 37:959–967
Tourneur J, Jouanin L, Muller JF, Caboche M (1985) A genetic approach to the study of the mechanism of action of auxin in tobacco. Susceptibility of an auxin resistant mutant to Agrobacterium transformation. ICN-UCLA Symp Mol Cell Biol Plant Genetics 35:791–797
Velten J, Willmitzer L, Leemans J, Ellis J, Deblaere R, van Montagu M, Schell J (1983) TR genes involved in agropine production. In: Puhler A (ed) Molecular genetics of the bacteria plant interaction, Springer, Berlin, Heidelberg, pp 455–462
Vilaine F, Casse-Delbart F (1987) Independent induction of transformed roots by the TL and TR regions of the Ri plasmid of agropine type Agrobacterium rhizogenes. Mol Gen Genet 206:17–23
Wang K, Herrela-Estrella L, van Montagu M, Zambryski P (1984) Right 25 bp terminus sequence of the nopaline T-DNA is essential for and determines direction of DNA transfer from Agrobacterium to the plant genome. Cell 38:455–462
White FF, Ghidossi G, Gordon MP, Nester EW (1982) Tumor induction by Agrobacterium rhizogenes involves the transfer of plasmid DNA to the plant genome. Proc Natl Acad Sci USA 79:3193–3197
White FF, Garfinkel DJ, Huffman GA, Gordon MP, Nester EW (1983) Sequences homologous to Agrobacterium rhizogenes T-DNA in the genomes of infected plants. Nature 301:348–350
White FF, Taylor BH, Huffman GA, Gordon MP, Nester EW (1985) Molecular and genetic analysis of the transferred DNA regions of the root inducing plasmid of Agrobacterium rhizogenes. J Bacteriol 164:33–44
Willmitzer L, Sanchez-Serrano J, Bushfeld E, Schell J (1982) DNA from Agrobacterium rhizogenes is transferred to and expressed in axenic hairy root plant tissues. Mol Gen Genet 186:16–22
Author information
Authors and Affiliations
Additional information
Communicated by J. Schell
Rights and permissions
About this article
Cite this article
Jouanin, L., Guerche, P., Pamboukdjian, N. et al. Structure of T-DNA in plants regenerated from roots transformed by Agrobacterium rhizogenes strain A4. Mol Gen Genet 206, 387–392 (1987). https://doi.org/10.1007/BF00428876
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00428876