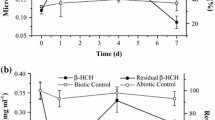
Abstract
Two chiral metabolites of the hexachlorinated cyclodien insecticide endosulfan, C9H6Cl6O3S, the endosulfan hydroxyether and the endosulfan lactone, were separated into their enantiomers using lipophilic β-cyclodextrin derivatives in chiral high-resolution gas chromatography (CHRGC). We separated the hydroxyether on heptakis-(2,3,6-tri-O-pentyl)-β-cyclodextrin (LIPODEX-C) and the endosulfan lactone on heptakis-(3-O-acetyl-2,6-di-O-pentyl)-β-cyclodextrin (LIPODEX-D). We also investigated the enantioselective formation and transformation of these two metabolites by soil organisms. To approximate real world conditions of microbiological transformation, incubation experiments with mixed cultures of soil microorganisms were carried out. Significant differences were observed in the transformation experiments under aerobic and anaerobic conditions. The endosulfan hydroxyether (ESH) is not formed enantioselectively from prochiral endosulfan diol (ESD) in the aerobic transformation pathway. The hydroxyether itself is enantioselectively converted to the endosulfan lactone (ESL) as a major pathway only under aerobic conditions. Corresponding enantiomers of endosulfan hydroxyether and endosulfan lactone with the same absolute configuration could be assigned. The lactone enantiomers are stereoselectively formed via the hydroxyether as a minor pathway under anaerobic conditions. While the first eluting lactone enantiomer was more abundant in the aerobic experiment, it was the second eluting under anaerobic conditions. Four major so far unidentified metabolites were detected in the anaerobic incubations of both the endosulfan hydroxyether and the endosulfan diol.
Similar content being viewed by others
References
Cotham EW, Bidleman TF (1989) J Agric Food Chem 37:824–828
Goebel H, Gorbach S, Knauf W, Rimpau RH, Hüttenbach H (1982) Res Rev 83:136–137
Worthing R, Hance RJ (1991) The pesticide manual — A world compendium, 9th edn. British Crop Protection Council, Farnham, pp 332–333
El Zorgani GA, Omer MEH (1974) Bull Env Contam Toxicol 12:182–185
World Health Organisation (1984) Endosulfan. Environmental Health Criteria, Geneva
Domsch KH (1992) Pestizide im Boden — Mikrobieller Abbau und Nebenwirkungen auf Mikroorganismen. VCH, Weinheim, pp 128–129
Burgoyne TW, Hites RA (1993) Environ Sci Technol 279:910–914
Cürten B (1994) Diploma Thesis, University of Ulm
Schreitmüller J (1994) Dissertation, University of Ulm
Buchert H, Class T, Ballschmiter K (1989) Fresenius Z Anal Chem 333:211–217
Perscheid M, Schlüter H, Ballschmiter K (1973) Z Naturforsch 28c:761–763
Maier-Bode H (1968) Res Rev 22:17–23
Ballschmiter K, Tölg G (1966) Angew Chemie 78:775–776
Schuphan I, Ballschmiter K (1968) Z Naturforsch 23B:701–706
Martens R (1977) Bull Environ Contam Toxicol 17:438–446
Schuphan I, Ballschmiter K (1972) Nature 237:100–101
Schuphan I, Ballschmiter K (1972) Z Pflanzenkrank Pflanzenschutz 79:23–26
Rückert W, Ballschmiter K (1972) Fresenius Z Anal Chemie 259:188–190
König WA, Icheln D, Runge T, Pfaffenberger B, Ludwig P, Hühnerfuss H (1991) JHRC 14:530–535
König WA (1989) Nachr Chem Tech Lab 37:471–476
Schurig V, Nowotny H (1990) Angew Chem 102:969–1108
Mössner S, Spraker TR, Becker P, Ballschmiter K (1992) Chemosphere 24:1171–1180
Müller MD, Schalbach M, Oehme M (1992) Environ Sci Technol 26:566–569
Faller J, Hühnerfuss H, König WA, Krebber R, Ludwig P (1991) Environ Sci Technol 25:676–678
Buser H, Müller MD (1992) Environ Sci Technol 26:1533–1540
Buser H, Müller MD (1992) Anal Chem 64:3168–3175
Schneider M (1993) Diploma Thesis, University of Ulm
Feichtinger H, Linden HW (1967) Chem Ber 100:855–862
Miles JRW, Moy P (1979) Bull Environ Contam Toxicol 23:13–19
Izumi Y, Tai A (1977) Stereodifferentiating reactions. Kodansha Scientific Books, Tokyo
Rückert W, Ballschmiter K (1973) Z Naturforsch 28c:107–112
Schuphan I, Ballschmiter K (1972) Fresenius Z Anal Chem 259:25–28
Author information
Authors and Affiliations
Additional information
Dedicated to Professor Dr. Dr. h.c. mult. J. F. K. Huber on the occasion of his 70th birthday
Rights and permissions
About this article
Cite this article
Schneider, M., Ballschmiter, K. Transformation experiments with two chiral endosulfan metabolites by soil microorganisms — CHIRAL HRGC on lipophilic cyclodextrin derivatives. Fresenius J Anal Chem 352, 756–762 (1995). https://doi.org/10.1007/BF00323060
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00323060