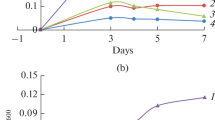
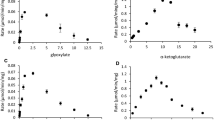
Abstract
The obligate anaerobe Eubacterium acidaminophilum metabolized the glycine derivatives sarcosine (N-monomethyl glycine) and betaine (N-trimethyl glycine) only by reduction in a reaction analogous to glycine reductase. Using formate as electron donor, sarcosine and betaine were stoichiometrically reduced to acetate and methylamine or trimethylamine, respectively. The N-methyl groups of the cosubstrates or of the amines produced were not transformed to CO2 or acetate. Under optimum conditions (formate/acceptor ratio of 1 to 1.2, 34°C, pH 7.3) the doubling times were 4.2 h on formate/sarcosine and 3.6 h on formate/betaine. The molar growth yields were 8.15 and 8.5 g dry cell mass per mol sarcosine and betaine, respectively. The assays for sarcosine reductase and betaine reductase were optimized in cell extracts; NADPH was preferred as physiological electron donor compared to NADH, dithioerythritol was used as artificial donor; no requirements for AMP and ADP could be detected. Growth experiments mostly revealed diauxic substrate utilization pattern using different combinations of glycine, sarcosine, and betaine (plus formate) and inocula from different precultures. Glycine was always utilized first, what coincided with the presence of glycine reductase activity under all growth conditions except for serine as substrate. Sarcosine reductase and betaine reductase were only induced when E. acidaminophilum was grown on sarcosine and betaine, respectively. Creatine was metabolized via sarcosine. [75Se]-selenite labeling revealed about the same pattern of predominant labeled proteins in glycine-, sarcosine-, and betaine-grown cells.
Similar content being viewed by others
Abbreviations
- DTE:
-
dithioerythritol
- TES:
-
N-Tris (hydroxymethyl) methyl-2-amino-ethane sulfonic acid
References
AndreesenJR, GottschalkG, SchlegelHG (1970) Clostridium formicoaceticum nov spec. Isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol 72: 154–174
BarnardGF, AkhtarM (1979) Mechanistic and stereochemical studies on the glycine reductase of Clostridium sticklandii. Eur J Biochem 99:593–603
BlundenG, GordonSM, McLeanWFH, GuiryMD (1982) The distribution and possible taxonomic significance of quarternary ammonium and other Dragendorff-positive compounds in some genera of marine algae. Bot Mar 25:563–567
BradfordMM (1976) A rapid and sensitive method for quantitation of microgram quantities of protein utilizing the principle of protein dye-binding. Anal Biochem 72:248–254
BreznakJA, SwitzerJM, SeitzHJ (1988) Sporomusa termitida sp nov, an H2/CO2-utilizing acetogen isolated from termites. Arch Microbiol 150:282–288
CarruthersA, OldfieldJET, TeagueHJ (1960) The removal of interfering ions in the detection of betaine in sugar-beet juices and plant material. Analyst 85:272–275
ConeJE, Martin del RioR, DavisJN, StadtmanTC (1976) Chemical characterization of the selenoprotein component of clostridial glycine reductase: Identification of selenocysteine as the organoselenium moiety. Proc Natl Acad Sci USA 73:2659–2663
ConeJE, Martin del RioR, StadtmanTC (1977) Clostridial glycine reductase complex. Purification and characterization of the selenoprotein component. J Biol Chem 252:5337–5344
CsonkaLN (1989) Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev 53:121–147
DornM, AndreesenJR, GottschalkG (1978) Fermentation of fumarate and L-malate by Clostridium formicoaceticum. J Bacteriol 133:26–32
EnerothP, LindstedtG (1965) Thin-layer chromatography of betaines and other compounds related to carnitine. Anal Biochem 10:479–485
FinkelsteinJD, MartinJJ, HarrisJ (1988) Methionine metabolism in mammals. The methionine-sparing effect of cystine. J Biol Chem 263:11750–11754
FochtRL, SchmidtFH (1956) Colorimetric determination of betaine in glutamate process and liquor. J Agric Food Chem 4:579–585
FreudenbergW, AndreesenJR (1989) Purification and partial characterization of the glycine decarboxylase multienzyme complex from Eubacterium acidaminophilum. J Bacteriol 171:2209–2215
FreudenbergW, DietrichsD, LebertzH, AndreesenJR (1989a) Isolation of an atypically small lipoamide dehydrogenase involved in the glycine decarboxylase complex from Eubacterium acidaminophilum. J Bacteriol 171:1346–1354
FreudenbergW, HormannK, RiethM, AndreesenJR (1989b) Involvement of a selenoprotein in glycine, sarcosine, and betaine reduction by Eubacterium acidaminophilum. In: WendelA (ed) Selenium in biology and medicine. Springer, Heidelberg, pp 25–28
FreudenbergW, MayerF, AndreesenJR (1989c) Immunocytochemical localization of proteins P1, P2, P3 of glycine decarboxylase, and of the selenoprotein PA of glycine reductase, all involved in anaerobic glycine metabolism of Eubacterium acidaminophilum. Arch Microbiol 152:182–188
GalinskiEA, TrüperHG (1982) Betaine, a compatible solute in the extremely halophilic phototrophic bacterium Ectothiorhodospira halochloris. FEMS Microbiol Lett 13:357–360
Gauglitz U (1988) Anaerober mikrobieller Abbau von Kreatin, Kreatinin and N-Methylhydantoin. PhD thesis, Univ Göttingen
GenthnerBRS, DavisCL, BryantBP (1981) Features of rumen and sewage sludge strains of Eubacterium limosum, a methanol- and H2−CO2-utilizing species. Appl Environ Microbiol 42:12–19
GoodwinJF, StampwalaS (1973) Spectrophotometric quantification of glycine in serum and urine. Clin Chem 19:1010–1015
GorhamJ (1984) Separation of plant betaines and their sulphur analogues by cation-exchange high-performance liquid chromatography. J Chromatogr 287:345–351
GreenbergDM (1961) Biosynthesis of amino acids and related compounds. In: GreenbergDM (ed) Metabolic pathways. Academic Press, New York, pp 173–236
HeigenerH (1935) Verwertung von Aminosäuren als gemeinsame C- und N-Quelle durch bekannte Bodenbakterien nebst botanischer Beschreibung neu isolierter Betain- und Valin-Abbauer. Zentralbl Bakt Abt II 93:81–113
HeijthuijsenJHFG, HansenTA (1989) Betaine fermentation and oxidation by marine Desulfuromonas strains. Appl Environ Microbiol 55:965–969
IkutaS, MatuuraK, ImamuraS, MisakiH, HoriutiY (1977) Oxidative pathways of choline to betaine in the soluble fraction prepared from Arthrobacter globiformis. J Biochem 82:157–163
ImhoffJF (1986) Osmoregulation and compatible solutes in eubacteria. FEMS Microbiol Rev 39:57–66
KortsteeGJJ (1970) The aerobic decomposition of choline by micro-organisms. I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as the sole carbon and nitrogen source. Arch Mikrobiol 71:235–244
LaemmliUK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
LangE, LangH (1972) Spezifische Farbreaktion zum direkten Nachweis der Ameisensäure. Z Anal Chem 260:8–10
LarherF, JolivetY, BriensU, GoasU (1982) Osmoregulation in higher plants halophytes: organic nitrogen accumulation in glycine, betaine, and proline during the growth of Aster tripolinum and Sueda macrocarpa under saline conditions. Plant Sci Lett 24:201–210
LeveringPR, BinnemaDJ, VanDijkenJP, HarderW (1981) Enzymatic evidence for a simultaneous operation of two one-carbon assimilation pathways during growth of Arthrobacter P1 on choline. FEMS Microbiol Lett 12:19–25
LovittRW, KellDD, MorrisJG (1986) Proline reduction by Clostridium sporogenes is coupled to vectorial proton ejection. FEMS Microbiol Lett 36:269–273
MargolisJ, KenrickKG (1967) Polyacrylamide gel electrophoresis across a molecular sieve gradient. Nature 214:1334–1336
MöllerB, OssmerR, HowardBH, GottschalkG, HippeH (1984) Sporomusa, a new genus of Gram-negative anaerobic bacteria including Sporomusa sphaeroides spec. nov. and Sporomusa ovata spec. nov. Arch Microbiol 139:388–396
MöllerB, HippeH, GottschalkG (1986) Degradation of various amine compounds by mesophilic clostridia. Arch Microbiol 145:85–90
MüllerE, FahlbuschK, WaltherR, GottschalkG (1981) Formation of N,N-dimethylglycine, acetic acid and butyric acid from betaine by Eubacterium limosum. Appl Environ Microbiol 42:439–445
NakajimaM, ShirokaneY, MizusawaK (1980) A new amidino-hydrolase, methylguanidine amidinohydrolase from Alcaligenes sp N-42. FEBS Lett 110:43–46
Naumann E (1983) Methanbildung aus Betain über Trimethylamin als Zwischenprodukt. PhD thesis, Univ Göttingen
NaumannE, HippeH, GottschalkG (1983) Betaine: a new oxidant in the Stickland reaction and methanogenesis from betaine and L-alanine by a Clostridium sporogenes-Methanosarcina barkeri coculture. Appl Environ Microbiol 45:474–483
Rieth M (1987) Untersuchungen zur selenabhängigen Glycin-Reduktase aus Eubacterium acidaminophilum. PhD thesis, Univ Göttingen
SetoB (1980) The Stickland reaction. In: KnowlesCJ (ed) Diversity of bacterial respiratory chains, vol II. CRC Press, Boca Raton, pp 49–64
ShimizuS, KimJM, ShinmenY, YamadaH (1986) Evaluation of two alternative metabolic pathways for creatinine degradation in microorganisms. Arch Microbiol 145:322–328
SliwskowskiMX, StadtmanTC (1987) Purification and immunological studies of selenoprotein A of the clostridial glycine reductase complex. J Biol Chem 262:4899–4904
StadtmanTC (1970) Glycine reductase systems (Clostridium). Meth Enzymol 17A:959–966
StouthamerAH (1979) The search for correlation between theoretical and experimental growth yields. In: QuayleJR (ed) Microbial biochemistry, Intern Rev Biochem, vol 21. University Park Press, Baltimore, pp 1–47
ThauerRK, JungermannK, DeckerK (1977) Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev 41:100–180
VanEykHG, VermaatHJ, Leijnse-YbemaHJ, LeijnseB (1968) The conversion of creatinine by creatininase of bacterial origin. Enzymologia 34:198–202
WiddelF (1988) Microbiology and ecology of sulfate- and sulfur-reducing bacteria. In: ZehnderAJB (ed) Biology of anaerobic microorganisms. John Wiley & Sons, New York, pp 469–585
YanceyPH, ClarkME, HandSC, BowlusRD, SomeroGN (1982) Living with water stress: Evolution of osmolyte systems. Science 217:1214–1222
ZindelU, FreudenbergW, RiethM, AndreesenJR, SchnellJ, WiddelF (1988) Eubacterium acidaminophilum sp. nov., a versatile amino acid-degrading anaerobe producing or utilizing H2 or formate. Arch Microbiol 150:254–266
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hormann, K., Andreesen, J.R. Reductive cleavage of sarcosine and betaine by Eubacterium acidaminophilum via enzyme systems different from glycine reductase. Arch. Microbiol. 153, 50–59 (1989). https://doi.org/10.1007/BF00277541
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00277541