Abstract
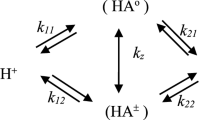
UV-visible and 13C NMR measurements described in the literature and our 31P NMR measurements support the following mechanism of proton transfer reactions in aqueous solutions of pyridoxamine phosphate: Only the tautomeric equilibrium between neutral form, A N, and zwitterion, A Z, which is analogous to the tautomeric equilibrium of 3-hydroxypyridine in aqueous solution, is important, and that equilibrium does not change upon the dissociation of the second phosphate proton. With these simplifying assumption, we have simulated the relaxation spectrum of the proton transfer reactions of pyridoxamine phosphate in water using parameters from analogous reactions and compared it with our ultrasound and temperature jump measurements. We have found that the relaxation process measured by the temperature jump experiment is mainly caused by the overall reaction A N=A Z (or A -N =A -Z ) and the ultrasound absorption at the isoelectric point between pK2 and pK3 is mainly caused by the overall reaction \(A^{\text{ + }} + {\text{x}} A_N^ - + \left( {1 - {\text{x}}} \right)A_Z^ - = {\text{y}}A_N + \left( {2 - {\text{y}}} \right)A_Z , 0 \leqq {\text{x}} \leqq {\text{1,}} {\text{0}} \leqq {\text{y}} \leqq 2\).
Similar content being viewed by others
References
Anderson FJ, Martell AE (1964) Pyridoxal phosphate: molecular species in solution. J Am Chem Soc 86:715–720
Asano T, Le Noble WJ (1978) Activation and reaction volumes in solution. Chem Rev 78:407–490
Bernasconi CF (1976) Relaxation kinetics. Academic Press, New York, pp 99–102
Bernasconi CF (ed) (1986) Techniques of chemistry, vol 6. John Wiley, New York, Part I and II
Christensen JJ, Hansen LD, Izatt RM (1976) Handbook of proton ionization heats. John Wiley, New York
Dubois JE, Dreyfus, ME (1978) Dynamic studies by chemical relaxation of prototropic equilibria in solution: recent advances. In: Laszlo P (ed) Protons and ions involved in fast dynamical phenomena. Elsevier, Amsterdam, pp 169–190
Eggers F (1967/1968) Eine Resonatormethode zur Bestimmung von Schallgeschwindigkeit und Dämpfung in geringen Flüssigkeitsmengen. Acustica 19:323–329
Eggers F, Funck T (1973) Ultrasonic measurements with milliliter liquid samples in the 0.5–100 MHz range. Rev Sci Instrum 44:969–977
Eigen M (1963) Protonenübertragung, Säure-Base-Katalyse und enzymatische Hydrolyse. Teil I: Elementarvorgänge. Angew Chem 75:489–508
Eigen M, DeMaeyer L (1974) Theoretical basis of relaxation spectrometry. In: Hammes GG (ed) Techniques of chemistry, vol 6, part II. John Wiley, New York, pp 63–146
Feldmann K, Gaugler BJM, Winkler H, Helmreich EJM (1975) Conformational transitions in glycogen phosphorylase reported by covalently bound pyridoxamine derivatives. Biochemistry 13:2222–2230
Hoffmann GW (1972) Entwicklung einer schnellen Temperatur-Sprung-Anlage und ihre Anwendung auf kooperative Basenpaarung. Dissertation, Universität Braunschweig
Jaworsky RJ, O'Leary MH (1979) 13C NMR spectroscopy of the vitamin B6 group. Methods Enzymol 62:436–454
Larson JW, Zeeb KG, Loren GL (1982) Heat capacities and volumes of dissociation of phosphoric acid (1st, 2nd, and 3rd), bicarbonate ion, and bisulfate ion in aqueous solution. Can J Chem 60:2141–2150
Metzler DE, Harris CM, Johnson RJ, Siano DB, Thomson JA (1973) Spectra of 3-hydroxypyridines. Band-shape analysis and evaluation of tautomeric equilibria. Biochemistry 12: 5377–5392
Morozov YV, Bazhulina NP, Karpeiskii MY, Ivanov VI, Kuklin AI (1966) Optical and luminescent properties of vitamin B6 and its derivatives. III. Pyridoxamine and pyridoxamine-5′-phosphate. Biofizika 11:228–236
Pörschke D (1976) Cable temperature jump apparatus with improved sensitivity and time resolution. Rev Sci Instrum 47: 1363–1369
Pörschke D, Eggers F (1972) Thermodynamics and kinetics of base-stacking interactions. Europ J Biochem 26:490–498
Reiber H (1972) Photochemical reactions of vitamin B6 compounds, isolation and properties of products. Biochim Biophys Acta 279:310–315
Reiter J, Beyer A, Potschka M, Schuster P, Winkler H, Ebeling H, Franck EU (1988) Proton transfer reactions of dibasic acids in aqueous solution: 3-hydroxypyridine and anthranilic acid. J Phys Chem (in press)
Schuster P, Tortschanoff K, Winkler H (1976) Protonenüber-tragungsreaktionen zweibasischer Säuren in wäßriger Lösung: 3-Hydroxypyridin. Z Naturforsch 31c:219–224
Schuster P, Wohlschann P, Tortschanoff K (1977) Dynamics of proton transfer in solution. In: Pecht I, Rigler R (eds) Chemical relaxation in molecular biology. Springer, Berlin Heidelberg New York, pp 107–190
Williams VR, Neilands JB (1954) Apparent ionization constants, spectral properties and metal chelation of the cotransaminases and related compounds. Arch Biochem Biophys 53:56–70
Yiv S, Lang J, Zana R (1978) Ultrasonic absorption in aqueous solutions of nucleotides and nucleosides. III. Kinetics of proton exchange in cytidine 5′-monophosphate and xanthosine 5′-monophosphate. In: Laszlo P (ed) Protons and ions involved in fast dynamical phenomena. Elsevier, Amsterdam, pp 311–322
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reiter, J., Schuster, P., Winkler, H. et al. Proton transfer reactions in aqueous solutions of pyridoxamine phosphate. Eur Biophys J 16, 219–229 (1988). https://doi.org/10.1007/BF00261264
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00261264


