Abstract
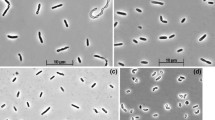
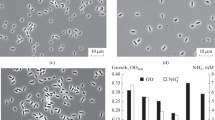
A new strictly anaerobic, gram-negative, nonsporeforming bacterium, Strain PerGlx1, was enriched and isolated from marine sediment samples with glyoxylate as sole carbon and energy source. The guanineplus-cytosine content of the DNA was 44.1±0.2 mol %. Glyoxylate was utilized as the only substrate and was stoichiometrically degraded to carbon dioxide, hydrogen, and glycolate. An acetyl-CoA and ADP-dependent glyoxylate converting enzyme activity, malic enzyme, and pyruvate synthase were found at activities sufficient for growth (≥0.25 U x mg protein-1). These findings allow to design a new degradation pathway for glyoxylate: glyoxylate is condensed with acetyl-CoA to form malyl-CoA; the free energy of the thioester linkage in malyl-CoA is conserved by substrate level phosphorylation. Part of the electrons released during glyoxylate oxidation to CO2 reduce a small fraction of glyoxylate to glycolate.
Similar content being viewed by others
References
Bartholomew JW (1962) Variables influencing results, and precise definition of steps in gram staining as a means of standardizing the results obtained. Stain Technol 37:139–155
Bergmever HU (1974) Methoden der enzymatischen Analyse, vol 1/2. Verlag Chemie, Weinheim/Bergstr, FRG
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brunner H, Chuard E (1886) Mittheilungen. Phytochemische Studien. Dtsch Chem Ges 19:595
Cord-Ruwisch R (1985) A quick method for the determination of dissolved and precipitated sulfides in cultures of sulfate-reducing bacteria. J Microbiol Methods 4:33–36
DeLey J (1970) Reexamination of the association between melting point, buoyant density, and the chemical base composition of deoxyribonucleic acid. J Bacteriol 101:738–754
Diekert GB, Thauer RK (1978) Carbon monoxide oxidation by Clostridium thermoaceticum and Clostridium formicoaceticum. J Bacteriol 136:597–606
Dimroth P (1981) Characterization of a membrane-bound biotincontaining enzyme: oxaloacetate decarboxylase from Klebsiella aerogenes. Eur J Biochem 115:353–358
Dixon GH, Kornberg HL (1959) Assay methods for key enzymes of the glyoxylate cycle. Biochem J 72:3p
Dörner C, Schink B (1990) Clostridium homopropionicum sp. nov., a new strict anaerobe growing with 2-, 3-, or 4-hydroxybutyrate. Arch Microbiol 154:342–348
Friedrich M, Laderer U, Schink B (1991) Fermentative degradation of glycolic acid by defined syntrophic cocultures. Arch Microbiol 156:398–404
Gottschalk G (1986) Bacterial metabolism, 2nd edn. Springer, New York Berlin Heidelberg
Gregersen T (1978) Rapid method for distinction of Gram-negative from Gram-positive bacteria. Eur J Appl Microbiol Biotechnol 5:123–127
Hansen RW, Hayashi JA (1962) Glycolate metabolism in Escherichia coli. J Bacteriol 83:679–687
International Union of Biochemistry (1984) Enzyme nomenclature 1984. Academic Press, Orlando, Florida
Kohn LD, Jakoby WB (1968) Tartaric acid metabolism VII. Crystal-line hydroxypyruvate reductase (d-glycerate dehydrogenase). J Biol Chem 243:2494–2499
Kornberg HL, Morris JG (1965) The utilization of glycollate by Micrococcus denitrificans: the beta-hydroxy-aspartate pathway. Biochem J 95:577–586
Kornberg HL, Sadler JR (1960) Microbial oxidation of glycollate via a dicarboxylic acid cycle. Nature 185:153–155
Lippmann EOvon (1894) Über organische Säuren aus Rübensaft. Dtsch Chem Ges 24:3303–3305
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Matthies C, Mayer F, Schink B (1989) Fermentative degradation of putrescine by new strictly anaerobic bacteria. Arch Microbiol 151:498–505
Odom JM, Peck HD (1981) Localisation of dehydrogenases, reductases and electron transfer components in the sulfate-reducing bacterium Desulfovibrio gigas. J Bacteriol 147:161–169
Pfennig N (1978) Rhodocyclus purpureus gen. nov. sp. nov., a ringshaped, vitamin B12-requiring member of the family Rhodospirillaceae. Int J Syst Bacteriol 28:283–288
Procházková L (1959) Bestimmung der Nitrate im Wasser. Z Anal Chem 167:254–260
Schink B (1984) Fermentation of 2,3-butanediol by Pelobacter carbinolicus sp. nov., and Pelobacter propionicus sp. nov. and evidence for propionate formation from C2 compounds. Arch Microbiol 137:33–41
Schink B (1991) Syntrophism among prokaryotes. In: The prokaryotes. Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds) Springer, New York Berlin Heidelberg (in press)
Schink B, Pfennig N (1982) Fermentation of trihydroxybenzenes by Pleobacter acidigallici gen. nov. spec. nov., a new strictly anaerobic, non-sporeforming bacterium. Arch Microbiol 133: 195–201
Spormann AM, Thauer RK (1988) Anaerobic acetate oxidation to CO2 by Desulfotomaculum acetoxidans. Demonstration of enzymes required for the operation of an oxidative acetyl-CoA/carbon monoxide dehydrogenase pathway. Arch Microbiol 150:374–380
Stams AJM, Kremer DR, Nicolay K, Weenk GH, Hensen TA (1984) pathway of propionate formation in Desulfobulbus propionicus. Arch Microbiol 139:167–173
Stewart R, Codd GA (1981) Glycollate and glyoxylate excretion by Sphaerocystis schroeteri (Chlorophyceae). Br Phycol J 16:177–182
Thauer RK (1988) Citric-acid Cycle, 50 years on. Eur J Biochem 176:497–508
Thauer RK, Jungermann K, Decker K (1977) Energy conservation of chemotrophic anaerobic bacteria. Bacteriol Rev 41:110–180
Tuboi S, Kikuchi G (1965) Enzymic cleavage of malyl-coenzyme A into acetyl-coenzyme A and glyoxylic acid. Biochim Biophys Acta 96:148–153
Widdel F, Kohring GW, Mayer F (1983) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. III. Characterization of the filamentous gliding Desulfonema limicola gen. nov. sp. nov., and Desulfonema magnum sp. nov. Arch Microbiol 134:286–294
Widdel F, Pfennig N (1981) Studies on dissimilatory sulfate-reducing bacteria that decompose fatty acids. I. Isolation of new sulfate reducing bacteria enriched with acetate from saline environments. Description of Desulfobacter postgatei gen. nov., sp. nov. Arch Microbiol 129:395–400
Widdel F, Pfennig N (1984) Dissimilatory sulfate- or sulfur reducting-bacteria. In: Krieg NR, Holt JG (eds) Bergey's manual of systematic bacteriology, IXth edn, vol 1. Williams & Wilkins, Baltimore London
Yamada EW, Jakoby WB (1958) Enzymatic utilization of acetylenic compounds. I. An enzyme converting acetylendicarboxylic acid to pyruvate. J Biol Chem 233:706–711
Zeltich I (1953) Oxidation and reduction of glycolic and glyoxylic acids in plants. J Biol Chem 201:719–726
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Friedrich, M., Schink, B. Fermentative degradation of glyoxylate by a new strictly anaerobic bacterium. Arch. Microbiol. 156, 392–397 (1991). https://doi.org/10.1007/BF00248716
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00248716