Summary
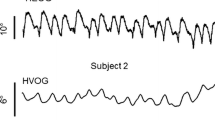
The decay of the slow phase velocity of post-rotatory (PRN) and optokinetic (OKAN) afternystagmus as a function of time was measured in Dutch rabbits after stimulation with velocity steps of 30, 60, and 150 °/s. The decays fitted linear functions very well, but only poorly exponential ones. Typical decay rates were 2–5 °/s2, with apparent time constants (defined by decay to 37% of initial velocity) in the order of 10–20 s. Within one animal, the decays of OKAN and PRN with similar initial velocities were indistinguishable. With sinusoidal oscillation, the time constant of the vestibulo-ocular reflex — estimated from phase lead — was only 2–3 s, and probably similar to the cupular time constant. In general, time constants increased when eye velocities increased. This indicates that the vestibulo-ocular reflex of the rabbit behaves as a non-linear system. A velocity storage system with a constant discharge rate is postulated as a main non-linear element. This would introduce a linear decay of velocity as well as a threshold for velocity. This storage system would be common to both vestibulo-ocular and optokinetic reflexes.
Similar content being viewed by others
References
Baarsma EA, Collewijn H (1974) Vestibulo-ocular and optokinetic reactions to rotation and their interaction in the rabbit. J Physiol (Lond) 238: 603–625
Batini C, Ito M, Kado RT, Jastreboff PJ, Miyashita Y (1979) Interaction between the horizontal vestibulo-ocular reflex and optokinetic response in rabbits. Exp Brain Res 37: 1–15
Blair S, Gavin M (1979) Response of the vestibulo-ocular reflex to differing programs of acceleration. Invest Ophthalmol 18: 1086–1090
Blanks RHI, Estes MS, Markham CH (1975) Physiological characteristics of vestibular first-order canal neurons in the cat. II. Response to constant angular acceleration. J Neurophysiol 38: 1250–1268
Buettner UW, Büttner U, Henn V (1978) Transfer characteristics of neurons in vestibular nuclei of the alert monkey. J Neurophysiol 41: 1614–1628
Büttner U, Waespe W, Henn V (1976) Duration and direction of optokinetic afternystagmus as a function of stimulus exposure time in the monkey. Arch Psychiat Nervenkr 222: 281–291
Cohen B, Matsuo V, Raphan T (1977) Quantitative analysis of the velocity characteristics of optokinetic nystagmus and optokinetic afternystagmus. J Physiol (Lond) 270: 321–344
Collewijn H (1969) Optokinetic eye movements in the rabbit. Input-output relations. Vision Res 9: 117–132
Collewijn H (1972) An analog model of the rabbit's optokinetic system. Brain Res 36: 71–88
Collewijn H (1976) Impairment of optokinetic (after-)nystagmus by labyrinthectomy in the rabbit. Exp Neurol 52: 146–156
Collewijn H (1977) Eye- and head movements in freely moving rabbits. J Physiol (Lond) 266: 471–498
Fernandez C, Goldberg JM (1971) Physiology of peripheral neurons innervating semicircular canals of the squirrel monkey. II. Response to sinusoidal stimulation and dynamics of peripheral vestibular system. J Neurophysiol 34: 661–675
Groen JJ (1957) Cupulometry. Laryngoscope 67: 894–905
Igarashi M, Takahashi M, Homick JL (1978) Optokinetic afternystagmus and postrotatory nystagmus in squirrel monkey. Acta Otolaryngol [Stockh] 85: 387–396
Jung R (1948) Die Registrierung des postrotatorischen und optokinetischen Nystagmus und die optisch-vestibuläre Integration beim Menschen. Acta Otolaryngol [Stockh] 36: 199–202
Koenig E, Allum JHJ, Dichgans J (1978) Visual-vestibular interaction upon nystagmus slow phase velocity in man. Acta Otolaryngol [Stockh] 85: 397–410
Landers PH, Taylor A (1975) Transfer function analysis of the vestibulo-ocular reflex in the conscious cat. In: Lennerstrand G, Bach-y-Rita P (eds) Basic mechanisms of ocular motility and their clinical implications. Pergamon Press, Oxford, pp 505–508
Mowrer OH (1937) The influence of vision during bodily rotation upon the duration of post-rotational vestibular nystagmus. Acta Otolaryngol [Stockh] 25: 351–364
Niven JI, Hixson WC, Correia MJ (1965) An experimental approach to the dynamics of the vestibular mechanisms. In: The role of the vestibular organs in the exploration of space. NASA, Washington DC (NASA SP-77)
Raphan T, Cohen B, Matsuo V (1977) A velocity-storage mechanism responsible for optokinetic nystagmus (OKN), optokinetic afternystagmus (OKAN) and vestibular nystagmus. In: Baker R, Berthoz A (eds) Control of gaze by brainstem neurons. Elsevier, Amsterdam, pp 37–47
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35: 229–248
Robinson DA (1976) Adaptive gain control of vestibulo-ocular reflex by the cerebellum. J Neurophysiol 39: 954–969
Robinson DA (1977) Vestibular and optokinetic symbiosis. An example of explaining by modelling. In: Baker R, Berthoz A (eds) Control of gaze by brainstem neurons. Elsevier, Amsterdam, pp 49–58
Skavenski AA, Robinson DA (1973) Role of abducence neurons in vestibulo-ocular reflex. J Neurophysiol 36: 724–738
Steinhausen W (1933) Über die Beobachtung der Cupula in den Bogengangsampullen des Labyrinths des lebenden Hechts. Pfluegers Arch 232: 500–512
Ter Braak JWG (1976) Untersuchungen über optokinetischen Nystagmus. Arch Neerl Physiol 21: 309–376
Van Egmond AAJ, Groen JJ, Jongkees LBW (1949) The mechanics of the semicircular canal. J Physiol (Lond) 110: 1–17
Waespe W, Henn V (1977a) Neuronal activity in the vestibular nuclei of the alert monkey during vestibular and optokinetic stimulation. Exp Brain Res 27: 523–538
Waespe W, Henn V (1977b) Vestibular nuclei activity during optokinetic after-nystagmus (OKAN) in the alert monkey. Exp Brain Res 30: 323–330
Waespe W, Henn V (1979) Motion information in the vestibular nuclei of alert monkeys. Visual and vestibular input vs. optomotor output. In: Granit R, Pompeiano O (eds) Reflex control of posture and movement. (Progress in Brain Research, vol 50) Elsevier, Amsterdam, pp 683–693
Winterson BJ, Collewijn H, Van der Steen J (1979) Postrotatory nystagmus, optokinetic afternystagmus and the vestibuloocular response in Dutch-belted rabbits. Neurosci Abstr 9: 1317
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Collewijn, H., Winterson, B.J. & van der Steen, J. Post-rotatory nystagmus and optokinetic after-nystagmus in the rabbit linear rather than exponential decay. Exp Brain Res 40, 330–338 (1980). https://doi.org/10.1007/BF00237798
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00237798