Summary
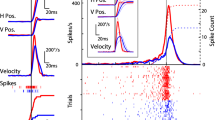
The integrity of the paramedian pontine reticular formation (PPRF) is necessary for the generation of rapid eye movements. The main saccade-related population is of the burst type with latencies between 0 and 40 ms preceding a saccade, and they can be divided into medium- and long-lead burst neurons. Burst neurons have predominantly spatially coded movement fields in the rostral PPRF, while in the caudal PPRF they increase their burst strength in temporal coding approximately in the pulling directions of extraocular eye muscles (i.e. almost horizontal or vertical). Both neuronal populations have ipsilateral on-directions and contain longlead burst neurons. In a quantitative analysis the firing patterns of long-lead burst neurons are compared to those of medium-lead burst neurons, which form the predominant output of the saccadic pulse generator to the motoneurons.
The firing patterns of temporally coded long-lead bursters are similar to those of medium-lead bursters, except for earlier on-latencies, larger statistical fluctuations, and specializations for small or large saccades in oblique directions. The spatially coded burst neurons form a motor map of saccadic vectors. The diameter of their movement field is often about the size of the saccade vector, and they encode saccadic onset and duration. These results are consistent with a model for visual saccades in eye displacement coordinates, where the spatio-temporal recoding of horizontal eye movements is effected by long-lead burst neurons in the PPRF.
Similar content being viewed by others
References
Bahill AT, Clark MR, Stark L (1975) The main sequence, a tool for studying human eye movements. Math Biosci 24: 191–204
Baker R, Spencer R, Evinger C (1982) Structure-function study in the oculomotor system. In: Lennerstrand G, Zee DS, Keller EL (eds) Functional basis of ocular motility disorders. Pergamon Press, Oxford, pp 277–280
Bender MB, Shanzer S (1964) Oculomotor pathways defined by electric stimulation and lesions in the brainstem of the monkey. In: Bender MB (ed) The oculomotor system. Harper & Row, New York, pp 81–140
Bizzi E (1968) Discharge of frontal eye field neurons during saccadic and following eye movements in unanesthetized monkeys. Exp Brain Res 6: 69–80
Büttner U, Büttner-Ennever JA, Henn V (1977) Vertical eye movement related activity in the rostral mesencephalic reticular formation of the alert monkey. Brain Res 130: 239–252
Cohen B, Henn V (1972) The origin of quick phases of nystagmus in the horizontal plane. Bibl Ophthalmol 82: 36–55
Goebel H, Komatsuzaki A, Bender MB, Cohen B (1971) Lesions of the pontine tegmentum and conjugate gaze paralysis. Arch Neurol 24: 431–440
Goldberg ME, Bushnell MC (1981) Behavioral enhancement of visual responses in monkey cerebral cortexx. II. Modulation in frontal eye fields specifically related to saccades. J Neurophysiol 46: 773–787
Grantyn A, Grantyn R (1982) Axonal patterns and sites of termination of cat superior colliculus neurons projecting in the tecto-bulbo-spinal tract. Exp Brain Res 46: 243–256
Hallet PE, Lightstone AD (1976) Saccadic eye movement towards stimuli triggered by prior saccades. Vision Res 16: 99–106
Harting JK (1977) Descending pathways from the superior colliculus: An autoradiographic analysis in the Rhesus monkey (macaca mulatta). J Comp Neurol 173: 583–612
Henn V, Cohen B (1976) Coding of information about rapid eye movements in the pontine reticular formation of alert monkeys. Brain Res 108: 307–325
Henn V, Hepp K (1981) Two-dimensional analysis of eye movements in oculomotor and premotor structures. In: Fuchs AF, Becker W (eds) Progress in oculomotor research. Elsevier/North-Holland, Amsterdam New York, pp 81–88
Henn V, Hepp K, Büttner-Ennever JA (1982) The primate oculomotor system II. Premotor system. Human Neurobiol 1: 87–95
Hepp K, Henn V (1979) Neuronal activity preceding rapid eye movements in the brainstem of the alert monkey. Prog Brain Res 50: 645–652
Hepp K, Henn V (1982a) Physiology of horizontal gaze. In: Lennerstrand G, Zee DS, Keller EL (eds) Functional basis of ocular motility disorders. Pergamon Press, Oxford, pp 247–255
Hepp K, Henn V (1982b) Spatio-temporal recoding in the generation of rapid eye movements. In: Roucoux A, Crommelinck M (eds) Physiological and pathological aspects of eye movements. W Junk Publ., The Hague, pp 319–323
Hepp K, Henn V, Jaeger J (1982) Eye movement related neurons in the cerebellar nuclei of the alert monkey. Exp Brain Res 45: 253–261
Hikosaka O, Igusa Y, Imai H (1980) Inhibitory connections of nystagmus-related reticular burst neurons with neurons in the abducens, prepositus hypoglossi and vestibular nuclei in the cat. Exp Brain Res 39: 301–311
Kaneko CRS, Evinger C, Fuchs AF (1981) Role of cat pontine burst neurons in generation of saccadic eye movements. J Neurophysiol 46: 387–408
Keller EL (1974) Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol 37: 316–332
Keller EL (1981) Brain stem mechanisms in saccadic control. In: Fuchs AL, Becker W (eds) Progress in oculomotor research. Elsevier/North Holland, Amsterdam, pp 57–62
Keller EL, Crandall WF (1981) Neural activity in the nucleus reticularis tegmenti pontis in the monkey related to eye movements and visual stimulation. Ann NY Acad Sci 374: 249–261
King WM, Lisberger SG, Fuchs AF (1976) Responses of fibers in medial longitudinal fasciculus (MLF) of alert monkeys during horizontal and vertical conjugate eye movements evoked by vestibular or visual stimuli. J Neurophysiol 39: 1135–1149
King WM, Fuchs AF (1979) Reticular control of vertical saccadic eye movements by mesencephalic burst neurons. J Neurophysiol 42: 861–876
Kömpf D, Pasik T, Pasik P, Bender MB (1979) Downward gaze in monkeys: Stimulation and lesion studies. Brain 102: 527–558
Lang W, Henn V, Hepp K (1982) Gaze palsies after selective pontine lesions in monkeys. In: Roucoux A, Crommelinck M (eds) Physiological and pathological aspects of eye movements. W Junk Publ., The Hague, pp 209–218
Leichnetz GR (1981) The prefrontal cortico-oculomotor trajectories in the monkey. J Neurol Sci 49: 387–396
Luschei ES, Fuchs AF (1972) Activity of brain stem neurons during eye movements of alert monkeys. J Neurophysiol 35: 445–461
Mays LE, Sparks DL (1980) Dissociation of visual and saccaderelated responses in superior colliculus neurons. J Neurophysiol 43: 207–232
McIlwain JT (1976) Large receptive fields and spatial transformations in the visual system. Int Rev Physiol 10: 223–248
Mohler CW, Wurtz RH (1976) Organization of monkey superior colliculus: Intermediate layer cells discharging before eye movements. J Neurophysiol 39: 722–744
Porter JD, Sparks DL (1982) Movement fields of pontine saccaderelated burst neurons. ARVO Abstr, p 104
Raphan T, Matsuo V, Cohen B (1979) Velocity storage in the vestibulo-ocular reflex arc (VOR). Exp Brain Res 35: 229–248
Raybourn MS, Keller EL (1977) Colliculoreticular organization in primate oculomotor system. J Neurophysiol 40: 861–878
Robinson DA, Fuchs AF (1969) Eye movements evoked by stimulation of frontal eye fields. J Neurophysiol 32: 637–648
Robinson DA (1972) Eye movements evoked by collicular stimulation in the alert monkey. Vision Res 12: 1795–1808
Robinson DA (1975) Oculomotor control signals. In: Lennerstrand G, Bach-y-Rita P (eds) Basic mechanisms of ocular motility and their clinical implications. Pergamon Press, Oxford, pp 337–374
Robinson DA (1981) Control of eye movements. In: Brookhart JM, Mountcastle VB (eds) Handbook of physiology. The nervous system II. Am Physiol Soc, Bethesda, pp 1321–1336
Schiller PH, Stryker MP (1972) Single-unit recording and stimulation in superior colliculus of the alert rhesus monkey. J Neurophysiol 35: 915–924
Schiller P, True SD, Conway JL (1980) Deficits in eye movements following frontal eye-field and superior colliculus ablations. J Neurophysiol 44: 1175–1189
Sparks DL (1978) Functional properties of neurons in the monkey superior colliculus: Coupling of neuronal activity and saccade onset. Brain Res 156: 1–16
Sparks DL, Mays LE (1980) Movement fields of saccade-related burst neurons in the monkey superior colliculus. Brain Res 190: 39–50
Sparks DL, Mays LE (1981) The role of the monkey superior colliculus in the control of saccadic eye movements: A current perspective. In: Fuchs AF, Becker W (eds) Progress in oculomotor research. Elsevier/North-Holland, Amsterdam, pp 137–144
Sparks DL, Mays LE (1983) The spatial localization of saccade targets. I. Compensation for stimulation-induced perturbations in eye position. J Neurophysiol 49: 45–63
Sparks DL, Porter JD (1983) The spatial localization of saccade targets. II. Activity of superior colliculus neurons preceding compensatory saccades. J Neurophysiol 49: 64–74
Van Gisbergen JAM, Robinson DA, Gielen S (1981) A quantitative analysis of saccadic eye movements by burst neurons. J Neurophysiol 45: 417–442
Wurtz RH, Goldberg ME (1972) Activity of superior colliculus in behaving monkey. III. Cells discharging before eye movements. J Neurophysiol 35: 575–586
Yoshida KM, McCrea R, Berthoz A, Vidal PP (1982) Morphological and physiological characteristics of inhibitory burst neurons controlling horizontal rapid eye movements in the alert cat. I Neurophysiol 48: 761–784
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Hepp, K., Henn, V. Spatio-temporal recoding of rapid eye movement signals in the monkey paramedian pontine reticular formation (PPRF). Exp Brain Res 52, 105–120 (1983). https://doi.org/10.1007/BF00237155
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00237155