Summary
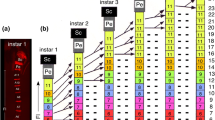
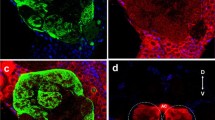
The postembryonic development of the antennal lobes of Periplaneta americana L. was examined with light- and electron-microscopical methods. There is no difference in the number of glomeruli and neurons in the antennal lobes of larval and adult animals. At hatching, the first larva already possesses the adult number of approximately 125 glomeruli and 500 to 560 deutocerebral neurons in the dorsolateral cell group of each antennal lobe. During postembryonic development the volume of the deutocerebral neurons increases three- to fourfold. The glomeruli of the first larva have about 7 % of the volume of the corresponding adult glomeruli. Since number, pattern, and size ratio of glomeruli (with the exception of the macroglomerulus) are constant in all larval stages and adult animals, it is possible to identify individual glomeruli. During the whole postembryonic development the ordinary glomeruli show a continuous volume increase, which parallels the increase in antennal sensory input. The macroglomerulus develops by way of special growth of two to four neuropil units, but not before the last three to four larval stages and only in males. Its growth precedes the formation of antennal pheromone receptors during the final molt; these receptors are known to project into the macroglomerulus. The development of the macroglomerulus in the last larval stages of the male may be caused by a genetically fixed growth program of specific deutocerebral neurons.
Similar content being viewed by others
References
Andres KH (1966) Über die Feinstruktur der Rezeptoren an Sinneshaaren. Z Zellforsch 75:339–365
Babu KS (1975) Development of the central nervous system of the spider Argiope aurantia (Lucas). J Morphol 146:325–342
Bate CM (1978) Development of sensory systems in arthropods. In: Marcus Jacobson (ed) Handbook of Sensory Physiology, Vol IX. Springer, Berlin Heidelberg New York, pp 1–53
Boeckh J, Boeckh V (1970) Threshold and odor specifity of pheromone-sensitive neurons in the deutocerebrum of Antheraea pernyi and A. polyphemus (Saturniidae). J Comp Physiol 132:235–242
Boeckh J, Sandri C, Akert K (1970) Sensorische Eingänge und synaptische Verbindungen im Zentralnervensystem von Insekten. Experimentelle Degeneration der antennalen Sinnesbahn im Oberschlundganglion von Fliegen und Schaben. Z Zellforsch 103:429–446
Boeckh J, Boeckh V, Kühn A (1977) Further data on the topography and physiology of central olfactory neurons in insects. Olfaction and Taste Vol. IV, Paris, pp 315–321
Bretschneider F (1924) Über die Gehirne des Eichenspinners und des Seidenspinners (Lasiocampaquercus und Bombyx mori L.). Jena Z Naturwiss 60:563–578
Campbell FL, Priestley JD (1970) Flagellar annuli of Blattella germanica (Dicty optera: Blattellidae). Changes in their numbers and dimensions during postembryonic development. Ann Entomol Soc Amer 63:81–88
Cambille I, Masson C (1980) The deutocerebrum of the cockroach Blaberus craniifer Burm. Spatial organization of the sensory gomeruli. J Neurobiol 11:135–157
Edwards JS (1969) Postembryonic development and regeneration of the insect nervous system. Advan Insect Physiol 6:97–137
Edwards JS, Palka J (1974) The cerci and abdominal giant fibres of the house cricket, Acheta domesticus. I. Anatomy and physiology of normal adults. Proc R Soc London 185:83–103
Ernst K-D, Boeck J, Boeckh V (1977) A neuroanatomical study on the organization of the central antennal pathways in insects. II. Deutocerebral connections in Locusta migratoria and Periplaneta americana. Cell Tissue Res 176:285–308
Goll W (1967) Strukturuntersuchungen am Gehirn von Formica. Z Morph Ökol Tiere 59:143–210
Gymer A, Edwards JS (1967) The development of the insect nervous system. I. An analysis of postembryonic growth in the terminal ganglion of Acheta domesticus. J Morphol 123:191–197
Haas H (1955) Untersuchungen zur Segmentbildung an der Antenne von Periplaneta americana. Roux' Archiv Entwicklungsmechanik 147:434–473
Hildebrand JG, Matsumoto SG, Camazine SM, Tolbert LP, Blank S, Ferguson H, Ecker V (1980) Organization and physiology of antennal centres in the brain of the moth Manduca sexta. In: Insect Neurobilogy and Pesticide Action (Neurotox 1979), London: Society of Chemical Industry, pp 375–382
Imms AD (1940) On the growth processes in the antennae of insects. Quart J Micros Sci 81:585–593
Jawlowski H (1948) Studies on the insect brain. Ann UMCS C Lublin 3:1–30
Johannson AS (1957) The nervous system of the milkweed bug Oncopeltus fasciatus Dallas (Heteroptera, Lygaeidae). Trans Am Ent Soc 83:119–183
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixation of high osmolality for use in electron microscopy. J Cell Biol 27:137A-138A
Landolt AM (1965) Elektronenmikroskopische Untersuchungen an der Perikaryenschicht der Corpora pedunculata der Waldameise (Formica lugubris Zett.) mit besonderer Berücksichtigung der Neuron-Glia-Beziehung. Z Zellforsch 66:701–736
Lawrence PA (1969) Polarity and patterns in the postembryonic development of insects. Adv Insect Physiol 7:197–266
Masson C, Strambi C (1977) Sensory antennal organization in an ant and a wasp. J Neurobiol 8:537–548
Murphey RK, Mendenhall B, Palka J, Edwards JS (1975) Deafferentation slows the growth of specific dendrites of identified giant interneurons. J Comp Neurol 159:407–418
Murphey RK, Matsumoto SG, Mendenhall B (1976) Recovery from deafferentation by cricket interneurons after reinnervation by their peripheral field. J Comp Neurol 169:335–346
Neder R (1975) Allometrisches Wachstum von Hirnteilen bei drei verschieden großen Schabenarten. Zool Jb Abt Anat Ontog 77:411–464
Nordlander RH, Edwards JS (1969) Postembryonic brain development in the monarch butterfly Danaus plexippus plexippus L. II. The optic lobes. Roux' Archiv Entwicklungsmechanik 163:197–220
Nordlander RH, Edwards JS (1970) Postembryonic brain development in the monarch butterfly Danaus plexippus plexippus L. III. Morphogenesis of centres other than the optic lobes. Roux' Archiv Entwicklungsmechanik 164:247–260
Palka J, Edwards JS (1974) The cerci and abdominal giant fibres of the house cricket Acheta domesticus. II. Regeneration and effects of chronic deprivation. Proc R Soc London B 185:105–121
Pflugfelder O (1937) Die Entwicklung der optischen Ganglien von Culex pipiens. Zool Anz 117:31–36
Pipa RL (1973) Proliferation, movement, and regression of neurons during the postembryonic development of insects. In: Young D (ed) Developmental Neurobiology of Arthropods. Cambridge University Press, London, pp 105–129
Ramón-Moliner E (1970) The Golgi technique. Contemporary research methods in neuroanatomy (Nauta WJH, Ebbesson SOE, eds). Springer Verlag, Berlin Heidelberg New York, pp 32–56
Reynolds ES (1963) The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol 17:208–212
Richardson KC, Jarett J, Finke EH (1960) Embedding in epoxy resins for ultrathin sectioning in electron microscopy. Stain Technol 35:313–323
Rospars J-P, Chambille I, Masson C (1979) Invariance morphologique de l'organisation glomerulaire du deutocerebron chez la Blatte Blaberus craniifer Burm. (Insecta, Dictyoptera). C R Hebd Seances Acad Sci, Paris, Ser D 288:1043–1046
Sanes JR, Hildebrand JG, Prescott DJ (1976) Differentiation of insect sensory neurons in the absence of their normal synaptic targets. Dev Biol 52:121–127
Sbrenna G (1971) Postembryonic growth of the ventral nerve cord in Schistocerca gregaria Forsk. (Orthoptera:Acrididae). Boll Zool 38:49–74
Schafer R, Sanchez TV (1973) Antennal sensory system of the cockroach Periplaneta americana, postembryonic development and morphology of the sense organs. J Comp Neurol 149:335–354
Schaller D (1978) Antennal sensory system of Periplaneta americana L. Distribution and frequency of morphologic types of sensilla and their sex-specific changes during postembryonic development. Cell Tissue Res 191:121–139
Selzer R (1979) Morphological and physiological identification of food odour specific neurons in the deutocerebrum of Periplaneta americana. J Comp Physiol 134:159–163
Smith DS, Treherne JE (1973) Functional aspects of the organization of the insect nervous system. In: Beament JWL, Treherne JE and Wigglesworth VB (eds) Advances in insect physiology I. Academic Press, New York
Suster PM (1933) Fühlerregeneration nach Ganglienextirpation bei Sphodromantis bioculata Burm. Zool Jb, Abt Allg Zool Physiol Tiere 53:41–48
Wigglesworth VB (1960) The nutrition of the central nervous system in the cockroach Periplaneta americana. The role of perineurium and glial cells in the mobilization of reserves. J Exp Biol 37:500–512
Author information
Authors and Affiliations
Additional information
Supported by the Deutsche Forschungsgemeinschaft (Scha 291/1)
Rights and permissions
About this article
Cite this article
Prillinger, L. Postembryonic development of the antennal lobes in Periplaneta americana L.. Cell Tissue Res. 215, 563–575 (1981). https://doi.org/10.1007/BF00233532
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00233532