Summary
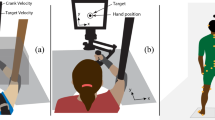
Two rhesus monkeys were trained to move a handle on a two-dimensional (2D) working surface in directions specified by a light at the plane. They first captured with the handle a light on the center of the plane and then moved the handle in the direction indicated by a peripheral light (cue signal). The signal to move (go signal) was given by turning off the center light. The following tasks were used: (a) In the non-delay task the peripheral light was turned on at the same time as the center light went off. (b) In the memorized delay task the peripheral light stayed on for 300 ms and the center light was turned off 450–750 ms later. Finally, (c) in the non-memorized delay task the peripheral light stayed on continuously whereas the center light went off 750–1050 ms after the peripheral light came on. Recordings in the arm area of the motor cortex (N= 171 cells) showed changes in single cell activity in all tasks. In both delay tasks, the neuronal population vector calculated every 20 ms after the onset of the peripheral light pointed in the direction of the upcoming movement, which was instructed by the cue light. Moreover, the strength of the population signal showed an initial peak shortly after the cue onset in both the memorized and non-memorized delay tasks but it maintained a higher level during the memorized delay period, as compared to the non-memorized task. These results indicate that the motor cortex is involved in encoding and holding in memory directional information concerning a visually cued arm movement and that these processes can be visualized using neuronal population vector analysis.
Similar content being viewed by others
References
Alexander GE, Crutcher MD (1990) Preparation for movement: neural representation of intended direction in three motor areas of the monkey. J Neurophysiol 64: 133–150
Bruce CJ, Goldberg ME (1985) Primate frontal eye field. I. Single neurons discharging before saccades. J Neurophysiol 53: 603–635
Chen D-F, Hyland B, Maier V, Palmeri A, Wiesendanger M (1991) Comparison of neural activity in the supplementary motor area and in the primary motor cortex in monkeys. Somatosensory Motor Res 8: 27–44
Clark MC, Marcario JK, Kettner RE (1991) Comparison of neuronal responses in the precentral cortex with EMG activity during an arm-movement sequence delay task. Soc Neurosci Abstr 17: 307
Crammond DJ, Kalaska JF (1991) Preparatory activity in premotor cortex during an instructed-delay period: relation to contra- and ipsilateral arm movements. Soc Neurosci Abstr 17: 308
Evarts EV (1981) Role of the motor cortex in voluntary movements in primates. In: Handbook of physiology. The nervous system, II. American Physiological Society, Bethesda, MD, pp 1083–1120
Funahashi S, Bruce CJ, Goldman-Rakic PS (1989) Mnemonic coding of visual space in the monkey's dorsolateral prefrontal cortex. J Neurophysiol 61: 331–349
Georgopoulos AP (1991) Higher order motor control. Ann Rev Neurosci 14: 361–377
Georgopoulos AP, Massey JT (1987) Cognitive spatial-motor processes. 1. The making of movements at various angles from a stimulus direction. Exp Brain Res 65: 361–370
Georgopoulos AP, Kalaska JF, Massey JT (1981) Spatial trajectories and reaction times of aimed movements: effects of practice, uncertainty, and change in target location. J Neurophysiol 46: 725–743
Georgopoulos AP, Kalaska JF, Caminiti R, Massey JT (1982) On the relations between the direction of two-dimensional arm movements and cell discharge in primate motor cortex. J Neurosci 2: 1527–1537
Georgopoulos AP, Caminiti R, Kalaska JF, Massey JT (1983) Spatial coding of movement: A hypothesis concerning the coding of movement direction by motor cortical populations. Exp Brain Res Suppl 7: 327–336
Georgopoulos AP, Kalaska JF, Crutcher MD, Caminiti R, Massey JT (1984) The representation of movement direction in the motor cortex: Single cell and population studies. In: Edelman GM, Cowan WM, Gall WE (eds) Dynamic aspects of neocortical function. Wiley, New York, pp 501–524
Georgopoulos AP, Schwartz AB, Kettner RE (1986) Neuronal population coding of movement direction. Science 233: 1416–1419
Georgopoulos AP, Kettner RE, Schwartz AB (1988) Primate motor cortex and free arm movements to visual targets in three-dimensional space. II. Coding of the direction of movement by a neuronal population. J Neurosci 8: 2928–2937
Georgopoulos AP, Crutcher MD, Schwartz AB (1989a) Cognitive spatial motor processes. 3. Motor cortical prediction of movement direction during an instructed delay period. Exp Brain Res 75: 183–194
Georgopoulos AP, Lurito JT, Petrides M, Schwartz AB, Massey JT (1989b) Mental rotation of the neuronal population vector. Science 243: 234–236
Ghez C, Hening W, Favilla M (1990) Parallel interacting channels in the initiation and specification of motor response features. Attention & Performance XIII: 265–293
Gnadt JW, Andersen RA (1988) Memory related motor planning activity in posterior parietal cortex of macaque. Exp Brain Res 70: 216–220
Hikosaka O, Wurtz RH (1983) Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol 49: 1268–1284
Hocherman S, Wise SP (1991) Effects of hand movement path on motor cortical activity in awake, behaving rhesus monkeys. Exp Brain Res 83: 285–302
Kettner RE, Marcario JK, Clark MC (1991) Population coding of movement direction in precentral cortex during a movement-sequence delay task. Soc Neurosci Abstr 17: 307
Lecas J-C, Requin J, Anger C, Vitton N (1986) Changes in neuronal activity of the monkey precentral cortex during preparation for movement. J Neurophysiol 56: 1680–1702
Lurito JL, Georgakopoulos T, Georgopoulos AP (1991) Cognitive spatial-motor processes. 7. The making of movements at an angle from a stimulus direction: studies of motor cortical activity at the single cell and population levels. Exp Brain Res 87: 562–580
Marcario JK, Kettner RE, Clark MC (1991) Simultaneously recorded activity in motor and premotor cortices of monkey during arm-movement sequences. Soc Neurosci Abstr 17: 307
Mardia KV (1972) Statistics of directional data. Academic Press, New York
Mauritz K-H, Wise SP (1986) Premotor cortex of the rhesus monkey: neuronal activity in anticipation of predictable environmental events. Exp Brain Res 61: 229–244
Moore BR (1980) A modification of the Rayleigh test for vector data. Biometrika 67: 175–180
Mountcastle VB, Reitboeck HJ, Poggio GF, Steinmetz MA (1991) Adaptation of the Reitboeck method of multiple microelectrode recording to the neocortex of the waking monkey. J Neurosci Methods 36: 77–84
Riehle A, Requin J (1989) Monkey primary motor and premotor cortex: single-cell activity related to prior information about direction and extent of an intended movement. J Neurophysiol 61: 534–549
Schwartz AB, Kettner RE, Georgopoulos AP (1988) Primate motor cortex and free arm movements to visual targets in three-dimensional space. I. Relations between single cell discharge and direction of movement. J Neurosci 8: 2913–2927
Smyrnis N, Ashe J, Taira M, Lurito JT, Georgopoulos AP (1991) Motor cortical cell activity in a memorized delay task. Soc Neurosci Abstr 17: 308
Snedecor GW, Cochran WG (1980) Statistical methods, 7th edn. Iowa State University Press, Ames, Iowa
Sokal RR, Rohlf FJ (1969) Biometry. Freeman, San Francisco
Mushiake H, Inase M, Tanji J (1991) Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol 66: 705–718
Winer BJ (1971) Statistical principles in experimental design, 2nd edition. Mc-Graw-Hill, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smyrnis, N., Taira, M., Ashe, J. et al. Motor cortical activity in a memorized delay task. Exp Brain Res 92, 139–151 (1992). https://doi.org/10.1007/BF00230390
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00230390