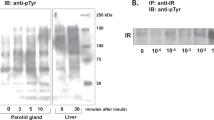
Abstract
While antibiotics are broadly used in dental and medical therapy, little attention has been directed towards the potential toxic side effects of antibiotics on tissue regeneration. Here we examined the effect of a quinolone antibiotic, pefloxacin (Rhone Poulenc) on rat parotid gland responses to chronic isoproterenol treatment. Groups of rats received injections of isoproterenol to induce glandular growth, saline (controls), pefloxacin, or isoproterenol and pefloxacin in combination. Parotid gland weight decreased significantly after pefloxacin treatment for 7 days as well as inhibiting glandular enlargement provoked by isoproterenol. The same trend was observed for the rates of DNA synthesis, with the incorporation of [3H]-thymidine in isoproterenol/pefloxacin-treated rats reduced to 49% of isoproterenol treatment alone levels. Saline-treated animals were 42% of the rate of [3H]-thymidine incorporation into DNA observed in isoproterenol treated rats. While isoproterenol treatment increased steady-state mRNA levels for fos, jun, myc, src, c-erbB-2, ras and topo II, inclusion of pefloxacin with the isoproterenol regimen blocked these increases. Pefloxacin treatment by itself did not alter proto-oncogene mRNA levels in the parotid gland. Glandular amylase activity was decreased in the pefloxacin treated group, while the combination of isoproterenol with pefloxacin did not decrease glandular amylase levels to the extent of that observed with β-agonist treatment alone. In acute experiments, pefloxacin significantly decreased the volume of saliva secreted by the parotid gland. These results suggest that quinolone-based antibiotics disturb the secretory function of the parotid gland and can inhibit cell proliferation and regeneration. (Mol Cell Biochem 165: 55–63, 1996)
Similar content being viewed by others
References
Wang JC: Interactions between DNAs and enzymes. The effect of superhelical turns. J Molec Biol 24: 87–94, 1974
Wang, JC: DNA topoisomerases. Ann Rev Biochem 54: 665–697, 1985
Hooper DC: Quinolone mode of action — new aspects. Drugs 45: (Suppl. 3): 8–14, 1993
Andriole VT: The future of quinolones. Drugs 45 (Suppl. 3): 1–7, 1993
Robinson MJ, Martin BA, Gootz TD, McGuirk PR, Osheroff N: Effects of novel fluoroquinolones on the catalytic activities of eukaryotic topoisomerase II: Influence of C-8 fluorine group. Antimicrob Agents Chemother 36: 751–756, 1992
Uemora T, Morikawa K, Yanagida M: The nucleotide sequence of the fission yeast DNA topoisomerase II gene: Structural and functional relationships to other DNA topoisomerases. EMBO J 5: 2355–2361, 1986
Yamashita Y, Arshizawa T, Morimoto M, Hosomi J, Nakano H: Antitumor quinolones with mammalian topoisomerase II mediated DNA cleavage activity. Cancer Res 52: 2818–2822, 1992
Sarkar M, Polk RE, Guzelian PS, Hunt C, Kames HT: In vitro effect of fluoroquinolones on theophylline metabolism in human liver microsomes. Antimicrob Agents Chemother 34: 594–599 1990
Eriksson H, Ridderstrale M, Degerman E, Ekholm D, Smith CJ, Manganiello VC, Belfrage P, Tornqvist H: Evidence for the key role of the adipose cGMP-inhibited cAMP phosphodiesterase in the antilipolytic action of insulin. Biochim Biophys Acta 1266: 101–107, 1995
Endoh M, Satoh H, Norota I, Hirano K, Hosokawa T: Subcellular mechanism of the positive ionotropic effect of a new quinolone derivative OPC-8490 on dog ventricular mycocardium. Heart Vessels 6: 158–167, 1991
Purushotham KR, Humphreys-Beher MG: The role of phosphotyrosine signalling pathway in parotid gland proliferation and function. Crit Rev Oral Biol 6: 119–131, 1995
Schneyer CA: Salivary gland changes after isoproterenol-induced enlargement. Am J Physiol 203: 232–236, 1962
Brown-Grant K: Enlargement of salivary glands in mice treated with isopropyl nor adrenaline. Nature 191: 1076–1078, 1961
Schneyer CA: β-adrenergic effects by autonomic agents on mitosis and hypertrophy in rat parotid. Proc Soc Exp Biol Med 131: 71–75, 1969
Quissell DO, Watson E, Dowd FJ: Signal transduction mechanisms involved in salivary gland regulated exocytosis. Crit Rev Oral Biol and Med 3: 83–107, 1992
Humphreys-Beher MG, Schneyer CA, Kidd VJ, Marchase RB: Isoproterenol-mediated parotid gland hypertrophy is inhibited by effectors of 4β-galactosyltransferase. J Biol Chem 262: 11706–11713, 1987
Purushotham KR, Dunn WA Jr, Schneyer CA, Humphreys-Beher MG: A novel mechanism for isoprenaline-stimulated proliferation of rat parotid gland acinar cells involving the EGF-receptor and cell surface galactosyltransferase. Biochem J 284: 767–776, 1992
Ciprofloxacin: Product Information Monograph. Compendium of Preclinical and Clinical Data. Marcel Dekker Inc, New York 37–45, 1988
Bradford M: A rapid and sensitive method for quantitation of microgram quantities of protein using the principle of protein-dye binding. Anal Biochem 72: 248–254, 1976
Nakagawa Y, Gammichia J, Purushotham KR, Schneyer CA, Humphreys-Beher MG: Epidermal growth factor activation of rat parotid gland adenylate cyclase and mediation by GTP-binding regulatory protein. Biochem Pharmacol 42: 2333–2340, 1991
Gilman AG: A protein binding assay for adenosine 3′:5′ cyclic monophosphate. Proc Natl Acad Sci USA 67: 305–312, 1970
Bernfeld P: Amylase, alpha and beta. Meth Enzymol. 1: 149–158, 1955
Chomezynski P, Sacchi N: Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 1162: 156–159, 1987
Shing Y, Christofori G, Hanahan D, Ono Y, Sasada R, Igarashi K, Folkman J, Betacellulin: A mitogen from pancreatic β cell tumors. Science 259: 1604–1607, 1993
Kerr M, Lee A, Wang PL, Purushotham KR, Chegini N, Yamamoto H, Humphreys Beher MG: Detection of insulin and insulin-like growth factors I and II in saliva and potential synthesis in the salivary glands of mice: Effect of type I diabetes mellitus. Biochem Pharmacol 49: 1521–1531, 1995
Lee S-W, Purushotham KR, Littlewood T, Evan G, Zelles T, Blazsek J, Nakagawa Y, Humphreys-Beher MG: Down-regulation of cellular proto-oncogenes during inhibition of rat parotid gland acinar cell proliferation. Biochem Biophvs Acta 1135: 115–122, 1992
Mirels L, Baum B, Kousvelari, E: Dissociation between c-fos gene expression and DNA synthesis in rat parotid glands. Arch Oral Biol 36: 511–515, 1989
Kousvelari E, Louis JM, Huang L-H, Curran T: Regulation of proto-oncogenes in rat parotid cells in vitro after stimulation of β-adrenergic receptors. Exp Cell Res 179: 194–203, 1988
Kawano M, Veno A, Ashida Y, Matsumoto N, Inoue H: Effect of sialagogues on ornathine decarboxylase induction and proto-oncogene expression in murine parotid gland. J Dent Res 71: 1885–1890, 1992
Kousvelari E, Yeh C-K, Mertz PM, Chinchetra M: Regulation of proto-oncogenes and salivary gland cell proliferation. Adv Dental Res 4: 61–68, 1990
Shaper NL, Harduin-Lepers A, Shaper JH: Male germ cell expression of murine β4-galactosyltransferase. A 796-base pair genomic region, containing two cAMP-responsive element (CRE)-like elements mediates male germ cell-specific expression in transgenic mice. J Biol Chem 269: 25165–25171, 1994
Quissel DO, Watson E, Dowd FJ: Signal transduction mechanisms involved in salivary gland regulated exocytosis. Crit Rev Oral Biol Med 3: 83–107, 1992
MacKay SLD, Bennett NT, Bland KI, Schultz GS: Growth factors, tumor suppressors, and cancer. Perspectives Gen Surg 2: 1–25, 1992
Herrera JL, Lyons MF II, Johnson LF: Saliva: Its role in health and disease. J Clin Gastroenterol 10: 569–578, 1988
Zelles T, Purushotham KR, Macauley SP, Oxford GE, and Humphreys-Beher MG: Saliva and growth factors: The fountain of youth within us all. J Dent Res 74: 1826–1832, 1995
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kelentey, B., Kerr, M., Tao, Z. et al. Inhibition of rat parotid gland growth response induced by chronic isoproterenol following treatment with quinolone antibiotics. Mol Cell Biochem 165, 55–63 (1996). https://doi.org/10.1007/BF00229745
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00229745