Summary
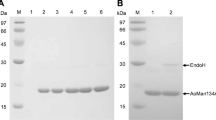
An endo-gb-1,4-mannanase cloned from “caldocellum saccharolyticum” and expressed in Escherichia coli was partially purified. The purification involved heat treatment, anion exchange and gel filtration. The mannanase was only active against mannan, glucomannans and galactoglucomannans and obeyed Michaelis-Menten kinetics on these substrates. The rate and extent of hydrolysis was dependent on the type of substrate. Galactomannans were not as readily depolymerized as the mannan and glucomannans investigated. The glucose content of the glucomannans did not affect the rate of hydrolysis and only slightly affected the extent. The molecular mass of the mannanase was estimated at 39 kDa. The pH and temperature optima were 6.5 and 80° C respectively. The mannanase was very thermostable with a half life of 48 min at 85° C and no loss in activity after 24 h at 70° C.
Similar content being viewed by others
References
Akino T, Kato C, Horikoshi K (1989) The cloned beta-mannanase gene from alkalophilic Bacillus sp. AM-001 produces two beta-mannanases in Escherichia coli. Arch Microbiol 152:10–15
Araujo A, Ward O (1990) Mannanase components from Bacillus pumilus. Appl Environ Microbiol 56:1954–1956
Aspinall GO (1970) Mannans, galactomannans and glucomannans. In: Polysaccharides. Pergamon, Toronto, pp 85–93
Clark TA, Mcdonald AG, Senior DJ, Mayers PR (1989) Mannanase and xylanase treatment of softwood chemical pulps: effects on pulp properties and bleachability. In: Kirk TK, Chang H-M (eds) Advances in biotechnology in the pulp and paper manufacture: Proceedings from the fourth international conference. Butterworths, Wellington, NZ, pp 153–168
Dekker RFH (1985) Biodegradation of the hemicelluloses. In: Higuchi T (ed) Biosynthesis and biodegradation of wood components. Academic Press, Toronto, pp 521–526
Dekker RFH, Richards GN (1976) Hemicellulases: their occurrence, purification, properties and mode of action. Adv Carbohydr Chem 32:277–352
Dixon M, Webb EC (1967) Enzyme kinetics. In: Dixon M, Webb EC (eds) Enzymes, 2nd edn. Longmans, London, pp 54–160
Donnison AM, Brockelsby CM, Morgan HW, Daniel RM (1989) The degradation of lignocellulosics by extremely thermophilic microorganisms. Biotechnol Bioeng 33:1495–1499
Harwood V (1973) Studies on the cell wall polysaccharides of Pinus radiata II. Structure of a glucomannan. Sven Papperstidn 76:377–379
Hudson R, Schofield L, Coolbear T, Daniel R, Morgan H (1991) Purification and properties of an aryl β-xylosidase from a cellulolytic extreme thermophile expressed in Escherichia coli. Biochem J in press
Kusakabe I, Takahashi R (1988) β-Mannanase from Streptomyces. Methods Enzymol. 160 Part A:611–614
Lever M (1973) Colorimetric and fluorometric carbohydrate determination with p-hydroxybenzoic acid hydrazide. Biochem Med 7:274–281
Love DR, Streiff MB (1987) Molecular cloning of a Beta-glucosidase from an extremely thermophilic anaerobe in E. coli and B. subtilis. Biotechnology 5:384–387
Luthi E, Berquist PL (1990) A β-xylosidase from the thermophile Caldocellum saccharolyticum. FEMS Microbiol Lett 67:291–294
Luthi E, Love DR, McAnulty J, Wallace C, Caughey PA, Saul D, Berquist PL (1990a) Cloning, sequence analysis, and expression of genes encoding xylan-degrading enzymes from the thermophile “Caldocellum saccharolyticum”. Appl Environ Microbiol 56:1017–1024
Luthi E, Jasmat NB, Berquist PL (1990b) Xylanase from the extremely thermophilic bacterium “Caldocellum saccharolyticum”: overexpression of the gene in Escherichia coli and characterization of the gene product. Appl Environ Microbiol 56:2677–2683
Luthi E, Jasmat NB, Grayling RA, Love DR, Berquist PL (1991) Cloning, sequence analysis, and expression in Escherichia coli of a gene coding for a β-mannanase from the extremely thermophilic bacterium “Caldocellum saccharolyticum”. Appl Environ Microbiol 57:694–700
McCleary BV (1979) Modes of action of β-mannanase enzymes of diverse origin on legume seed galactomannans. Phytochemistry 18:757–763
McCleary BV (1988) β-abetd-Mannanase. Methods Enzymol 160 Part A:596–610
Meder R, Ede RM, Bicho PA (1991) 1H and 13C NMR study of the products of enzymatic treatment of ivory nut mannan and Pinus radiata glucomannan. Carbohydrate Res in press
Park GG, Kusakabe I, Komatsu Y, Kobayashi G, Yasui T, Murakami K (1987) Purification and some properties of β-mannanase from Penicillin purpurogenum. Agric Biol Chem 51:2709–2716
Patchett ML, Neal TL, Schofield LR, Strange RC, Daniel RM, Morgan HW (1989) Heat treatment purification of thermostable cellulase and hemicellulase enzymes expressed in E. coli. Enzyme Microb Technol 11:113–115
Petersen GL (1977) A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem 83:346–356
Plant AR, Oliver JE, Patchett ML, Daniel RM, Morgan HW (1988) Stability and substrate specificity of a β-glucosidase from the thermophilic bacterium Tp8 cloned into E. coli. Arch Biochem Biophys 262:181–188
Ratto M, Poutanen K (1988) Production of mannan-degrading enzymes. Biotechnol Lett 10:661–664
Reynolds PH, Sissons CH, Daniel RM, Morgan HW (1986) Comparison of cellulolytic activities in Clostridium thermocellum and three thermophilic cellulolytic anaerobes. Appl Environ Microbiol 51:12–17
Schofield LR, Neal TL, Patchett ML, Strange RC, Daniel RM, Morgan HW (1988) The purification of cellulase and hemicellulase components from an extreme thermophile by cloning of enzymes into E. coli. Ann N Y Acad Sci 542:240–243
Sissons CH, Sharrock KR, Daniel RM, Morgan HW (1987) Isolation of cellulolytic anaerobic extreme thermophiles from New Zealand thermal sites. Appl Environ Microbiol 53:832–838
Shimizu K, Ishihara M (1983) Isolation and characterization of oligosaccharides from the hydrolyzate of larch wood glucomannan with endo-β-abetd-mannanase. Agric Biol Chem 47:949–955
Takahashi R, Kusakabe I, Kusama S, Sakuni Y, Murakami K, Maekawa A, Suzuki T (1984) Structures of glucomannan oligosaccharides from the hydrolytic products of konjac glucomannan produced by a β-mannanase from Streptomyces sp. Agric Biol Chem 48:2943–50
Talbot G, Sygusch J (1990) Purification and characterization of thermostable β-mannanase and α-galactosidase from Bacillus stearothermophilus. Appl Environ Microbiol 56:3505–3510
Timell TE (1967) Recent progress in the chemistry of wood hemicellulose. Wood Sci Technol 1:45–70
White CA, Kennedy JF (1986) Oligosaccharides. In: Chaplin M, Kennedy J (eds) Carbohydrate analysis, IRL Press, Washington, D.C., p 375
Widdel F, Kennedy G, Mayer F (1983) Studies on the dissimilatory sulphate-reducing bacteria that decompose fatty acids. III Characterisation of the filamentous gliding Desulfonema limicola gen. sp. nov. and Desulfonema magnum sp. nov. Arch Microbiol 134:286–294
Author information
Authors and Affiliations
Additional information
Offprint request to: H. W. Morgan
Rights and permissions
About this article
Cite this article
Bicho, P.A., Clark, T.A., Mackie, K. et al. The characterisation of a thermostable endo-β-1,4-mannanase cloned from “Caldocellum saccharolyticum”. Appl Microbiol Biotechnol 36, 337–343 (1991). https://doi.org/10.1007/BF00208153
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00208153