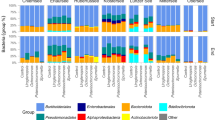
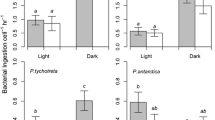
Abstract
Research on “microbial loop” organisms, heterotrophic bacteria and phagotrophic protists, has been stimulated in large measure by Pomeroy's seminal paper published in BioScience in 1974. We now know that a significant fate of bacterioplankton production is grazing by < 20-µm-sized flagellates. By selectively grazing larger, more rapidly growing and dividing cells in the bacterioplankton assemblage, bacterivores may be directly cropping bacterial production rather than simply the standing stock of bacterial cells. Protistan herbivory, however, is likely to be a more significant pathway of carbon flow in pelagic food webs than is bacterivory. Herbivores include both < 20-µm flagellates as well as > 20-µm ciliates and heterotrophic dinoflagellates in the microzooplankton. Protists can grow as fast as, or faster than their phytoplankton prey. Phototrophic cells grazed by protists range from bacterial-sized prochlorophytes to large diatom chains (which are preyed upon by extracellularly-feeding dinoflagellates). Recent estimates of microzooplankton herbivory in various parts of the sea suggest that protists routinely consume from 25 to 100% of daily phytoplankton production, even in diatom-dominated upwelling blooms. Phagotrophic protists should be viewed as a dominant biotic control of both bacteria and of phytoplankton in the sea.
Similar content being viewed by others
References
Andersen (1989) Functional biology of the choanoflagellate Diaphanoeca grandis Ellis. Mar Microb Food Webs 3:35–50
Azam F, Ammerman JW (1984) The cycling of organic matter by bacterio-plankton in pelagic marine ecosystems: microenvironmental considerations. In: Fasham MIR (ed) Flows of energy and materials in marine ecosystems: theory and practice. Plenum Press, New York, pp 345–360
Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Banse K (1982) Cell volumes, maximal growth rates of unicellular algae and ciliates, and the role of ciliates in the marine pelagial. Limnol Oceanogr 27:1059–1071
Banse K (1992) Grazing, temporal changes of phytoplankton concentrations, and the microbial loop in the open sea. In: Falkowski PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Plenum Press, New York, pp 409–440
Bjomsen PK, Kuparinen J (1991) Growth and herbivory by heterotrophic dinoflagellates in the South Ocean, studied by microcosm experiments. Mar Biol 109:397–405
Bockstahler KR, Coats WD (1993) Spatial and temporal aspects of mixotrophy in Chesapeake Bay dinoflagellates. J Protozool 40:49–60
Buck K, Bolt PA, Garrison DL (1990) Phagotrophy and fecal pellet production by an athecate dinoflagellate in Antarctic sea ice. Mar Ecol Prog Ser 60:75–84
Burkill PH, Mantoura RFC, Llewellyn CA, Owens NIP (1987) Microzooplankton grazing and selectivity of phytoplankton in coastal waters. Mar Biol 93:581–590
Burkill PH, Edwards ES, John AWG, Sleigh MA (1993) Microzooplankton and their herbivorous activity in the northeastern Atlantic Ocean. Deep-Sea Res 40:479–494
Burkill PH, Leakey RJG, Owens NIP, Mantoura RFC. (1993) Synnechococcus and its importance to the microbial food-web of the northwest Indian Ocean. Deep-Sea Res 40:773–782
Bursa AS (1961) The annual cycle at Igloolik in the Canadian Arctic. II. The phytoplankton. J Fish Res Bd Can 18:563–615
Campbell L, Carpenter EJ (1986) Estimating the grazing pressure of heterotrophic nanoplankton on Synechococcus spp. using the sea water dilution and selective inhibitor techniques. Mar Ecol Prog Ser 33:121–129
Campbell L, Vaulot D (1993) Photosynthetic picoplankton community structure in the subtropical North Pacific near Hawaii (station ALOHA). Deep-Sea Res 40:2043–2060
Capriulo GM, Sherr EB, Sherr BF (1991) Trophic behaviour and related community feeding activities of heterotrophic marine protists. In: Reid PC, Turley CM, Burkill PH (eds) Protozoa and their role in marine processes. (NATO ASI Series G, vol 25) Springer-Verlag, Berlin, pp 219–265
Caron DA, Goldman JC (1990) Protozoan nutrient regeneration. In: Capriulo GM (ed) Ecology of marine protozoa. Oxford Univ Press, New York, pp 283–306
Caron DA, Lim EL, Miceli G, Waterbury JB, Valois FW (1992) Grazing and utilization of chroococcoid cyanobacteria and heterotrophic bacteria by protozoa in laboratory cultures and a coastal plankton community. Mar Ecol Prog Ser 76:205–217
Chisholm SW (1992) Phytoplankton size. In: Falkowski PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Plenum Press, New York, pp 213–237
Chisholm SW, Olson RJ, Zettler ER, Goericke R, Waterbury JB, Welchmeyer NA (1988) A novel free-living prochlorophyte abundant in the ocean is euphotic zone. Nature 334:340–343
Choi JW, Peters J (1992) Effects of temperature on two psychrophilic ecotypes of a heterotrophic nanoflagellate, Paraphysomonas imperforata. Appl Environ Microbiol 58:593–599
Ducklow H (1983) Production and fate of bacteria in the oceans. BioScience 33:494–501
Fenchel T (1982) Ecology of heterotrophic microflageilates. II. Bioenergetics and growth. Mar Ecol Prog Ser 8:225–231
Fenchel T (1982) Ecology of heterotrophic microflagellates. IV. Quantitative occurrence and importance as bacterial consumers. Mar Ecol Prog Ser 9:35–42
Fuhrman JA, Azam F (1982) Thymidine incorporation as a measure of heterotrophic bacterial production in marine surface waters: evaluation and field results. Mar Biol 66:109–120
Gaines G, Elbrachter M (1987) Heterotrophic nutrition. In: Taylor FIR (ed) The biology of dinoflagellates. Blackwell Scientific, Oxford, pp 224–268
Gaines G, Taylor FIR (1984) Extracellular digestion in marine dinoflagellates. J Plank Res 6:1057–1061
Gallegos CL (1989) Microzooplankton grazing on phytoplankton in Rhode River, Maryland: nonlinear feeding kinetics. Mar Ecol Prog Ser 57:23–33
Gifford DJ (1988) Impact of grazing by microzooplankton in the Northwest Arm of Halifax Harbour, Nova Scotia. Mar Ecol Prog Ser 47:249–258
Gifford DJ (1991) The protozoan-metazoan trophic link in pelagic ecosystems. J Protozool 38:81–86
Goericke R, Repeta D (1992) The pigments of Prochlorococcus marinus: the presence of divinyl chlorophyll a and b in a marine procaryote. Limnol Oceanogr 37:425–434
Goldman JC (1984) Conceptual role for microaggregates in pelagic waters. Bull Mar Sci 35:462–476
Goldman JC, Caron DA (1985) Experimental studies on an omnivorous microflagellate: implications for grazing and nutrient regeneration in the marine microbial food chain. Deep-Sea Res 32:899–915
Goldman JC, Dennett MR, Gordin H (1989) Dynamics of herbivorous grazing by the heterotrophic dinoflagellate Oxyrrhis marina. J Plankton Res 11:391–407
Gonzalez JM, Sherr EB, Sherr BF (1990) Size-selective grazing on bacteria by natural assemblages of estuarine flagellates and ciliates. Appl Environ Microbiol 56:583–589
Gonzalez JM, Sherr BF, Sherr EB (1993) Digestive enzyme activity as a quantitative measure of protistan grazing: the acid lysozyme assay for bacterivory. Mar Ecol Prog Ser 100:197–206.
Gonzalez JM, Sherr EB, Sherr BF (1993) Differential feeding by marine flagellates on growing vs starving, and on motile vs nonmotile, bacterial prey. Mar Ecol Prog Ser 102:257–267
Haas LW, Webb KL (1979) Nutritional mode of several nonpigmented microflagellates from the York River estuary, Virginia. J Exp Mar Biol Ecol 39:125–134
Hansen PJ (1991) Quantitative importance and trophic role of heterotrophic dinoflagellates in a coastal pelagial food web. Mar Ecol Prog Ser 73:253–261
Heinbokel JF (1978) Studies on the functional role of tintinnids in the Southern California Bight. I. Grazing and growth rates in laboratory cultures. Mar Biol 47:191–197
Hobbie JE, Daley RJ, Jasper S (1977) Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol 33:1225–1228
Jacobsen DM (1993) Comparative ultrastructure of the food vacuoles of Dinophysis acuminata, Dinophysis rotundata, and Oxyphysis oxytoxoides. In: Smayda TJ, Shimizu Y (eds) Proc. 5th international conference on toxic marine phytoplankton. Elsevier, Amsterdam, pp 000–000
Jacobsen DM, Anderson, DM (1986) Thecate heterotrophic dinoflagellates: feeding behavior and mechanism. J Phycol 22:249–258
Jacobsen DM, Anderson DM (1993) Growth and grazing rates of Protoperidinium hirobis Abe, a thecate heterotrophic dinoflagellate. J Plankton Res 15:723–736
Johnson PM, Sieburth J.McN. (1979) Chroococcoid cyanobacteria in the sea: a ubiquitous and diverse phototrophic biomass. Limnol Oceanogr 24:928–935
Johnson PM, Sieburth J.McN. (1982) In situ morphology and occurrence of eucaryotic phototrophs of bacterial size in the picoplankton of estuarine and oceanic waters. J Phycol 18:318–327
Jonsson PR (1986) Particle size selection, feeding rates, and growth dynamics of marine planktonic oligotrichous ciliates (Ciliophora: Oligotrichina). Mar Ecol Prog Ser 33:265–277
Kuosa H (1991) Picoplanktonic algae in the northern Baltic Sea: seasonal dynamics and flagellate grazing. Mar Ecol Prog Ser 73:269–276
Landry MR, Hassett RP (1982) Estimating the grazing impact of marine micro-zooplankton. Mar Biol 67:283–288
Landry MR, Constantinou J, Kirstein JD, Allen C, Selph SE (1992) Phytoplankton growth and microzooplankton grazing in the equatorial Pacific Ocean. EOS 73:296
Lessard EJ (1991) The trophic role of heterotrophic dinoflagellates in diverse marine environments. Mar Microb Food Webs 5:49–58
Lessard EJ, Rivkin RB (1986) Nutrition of microzooplankton and macrozooplankton from McMurdo Sound. Antarct J US 21:187–188
Lessard EJ, Swift E (1985) Species-specific grazing rates of heterotrophic dinoflagellates in oceanic waters. Mar Biol 87:289–296
Lessard EJ, Swift E (1986) Dinoflagellates from the North Atlantic classified as phototrophic or heterotrophic by epifluorescence microscopy. J Plankton Res 8:1209–1215
Li WKW, Dickie PM, Irwin BD, Wood AM (1992) Biomass of bacteria, cyanobacteria, prochlorophytes, and photosynthetic eukaryotes in the Sargasso Sea. Deep-Sea Res 39:501–519
Longhurst AR (1989) Pelagic ecology: definition of pathways for material and energy flux. In: M. Michel Denis (ed) Oceanologie: actualite et prospective. Centre d'Oceanologie de Marseille, pp 263–288
McManus GB, Fuhrman JA (1988) Control of marine bacterioplankton populations: measurement and significance of grazing. Hydrobiologia 159:51–62
Miller CB, Frost BW, Booth B, Wheeler PA, Landry MR, Welschmeyer N (1991) Ecological processes in the subarctic Pacific: iron limitation cannot be the whole story. Oceanography 4:71–78
Monger BC, Landry MR (1992) Size-selective grazing by heterotrophic nanoflagellates: an analysis using live-stained bacteria and dual-beam flow cytometry. Arch Hydrobiol Beih Ergebn Limnol 37:173–185
Muller H, Geller W (1993) Maximum growth rates of aquatic ciliated protozoa: the dependence on body size and temperature reconsidered. Arch Hydrobiol 126:315–327
Nakamura Y, Yamazaki Y, Jiromi, J (1992) Growth and grazing of a heterotrophic dinoflagellate, Gyrodinium dominans, feeding on a red tide flagellate, Chattonella antiqua. Mar Ecol Prog Ser 82:275–279
Nothig E-M, von Bodungen B (1989) Occurrence and vertical flux of fecal pellets of probable protozoan origin in the Southeastern Weddell Sea (Antarctica). Mar Ecol Prog Ser 56:281–289
Olson RJ, Chisholm SW, Zettler ER, Altabet MA, Dusenberry JA (1990) Spatial and temporal distributors of prochlorophyte picoplankton in the North Atlantic Ocean. Deep-Sea Res 37:1033–1051
Ohman MD, Snyder RA (1991) Growth kinetics of the omnivorous oligotrich ciliate Strombidium sp. Limnol Oceangr 36:922–935
Pace ML (1988) Bacterial mortality and the fate of bacterial production. Hydrobiologia 159:41–50
Paranjape MA (1987) Grazing by microzooplankton in the eastern Canadian arctic in summer 1983. Mar Ecol Prog Ser 40:239–246
Paranjape MA (1990) Microzooplankton herbivory on the Grand Bank (Newfoundland, Canada): a seasonal study. Mar Biol 107:321–328
Platt T, Subba Rao DV, Irwin B (1983) Photosynthesis of picoplankton in the oligotrophic ocean. Nature 301:702–704
Pomeroy LR (1974) The ocean's food web, a changing paradigm. BioScience 24:499–504
Proctor LM, Fuhrman JA (1990) Viral mortality of marine bacteria and cyanobacteria. Nature 343:60–62
Rassoulzadegan F, Laval-Peuto M, Sheldon RW (1988) Partitioning of the food ration of marine ciliates between pico- and nanoplankton. Hydrobiologia 159:75–88
Raven JA (1986) Physiological consequences of extremely small size for autotrophic organisms in the sea. In: Platt T, Li WKW (eds) Photosynthetic picoplankton, Can Bull Fish Aquat Sci 214:1–70
Sanders RW, Porter KG (1988) Phagotrophic phytoflagellates. Adv Microb Ecol 10:167–192
Sanders RW, Caron DA, Berninger U-G (1992) Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: an inter-ecosystem comparison. Mar Ecol Prog Ser 86:1–14
Shapiro LP, Guillard RRL (1986) Physiology and ecology of the marine eukaryotic ultraplankton. In: Platt T, Li WKW (eds) Photosynthetic picoplankton. Can Bull Fish Aquat Sci 214:371–389
Sherr BF, Sherr EB (1991) Proportional distribution of total numbers, biovolume, and bacterivory among size classes of 2–20-µm nonpigmented marine flagellates. Mar Microb Food Webs 5:227–237
Sherr BF, Sherr EB, Pedros-Alio C (1989) Simultaneous measurement of bacterioplankton production and protozoan bacterivory in estuarine water. Mar Ecol Prog Ser 54:209–219
Sherr BF, Sherr EB, McDaniel J (1992) Effect of protistan grazing on the frequency of dividing cells in bacterioplankton assemblages. Appl Environ Microbiol 58:2381–2385
Sherr EB, Sherr BF (1988) Role of microbes in pelagic food webs: a revised concept. Limnol Oceanogr 33:1225–1227
Sherr EB, Sherr BF (1991) Planktonic microbes: tiny cells at the base of the ocean's food web. Trends in Ecol & Evol 6:50–54
Sherr EB, Sherr BF (1992) Trophic roles of pelagic protists: phagotrophic flagellates as herbivores. Arch Hydrobiol Beih Ergebn Linmol 37:165–172
Sherr EB, Sherr BF, McDaniel J (1991) Clearance rates of < 6-µm fluorescently-labeled algae (FLA) by estuarine protozoa: potential grazing impact of flagellates and ciliates. Mar Ecol Prog Ser 69:81–92
Sieburth J.McN. (1984) Protozoan bacterivory in pelagic marine waters. In: Hobbie JE, Williams PJleB (eds) Heterotrophic activity in the sea. Plenum Press, New York, pp 405–444
Sieburth J.McN., Smetacek V, Lenz J (1978) Pelagic ecosystem structure: heterotrophic compartments of plankton and their relationship to plankton size fractions. Limnol Oceanogr 33:1225–1227
Smetacek V (1981) The annual cycle of protozooplankton in Kiel Bight. Mar Biol 63:1–11
Steele JH (1974) The structure of marine ecosystems. Harvard University Press, Cambridge, Mass.
Stoecker DK, Capuzzo JMcD (1990) Predation on protozoa: its importance to zooplankton. J Plankton Res 12:891–908
Stoecker DK, Davis LH, Provan A (1983) Growth of Favella sp. (Ciliata: Tintinnina) and other microzooplankters in cages incubated in situ and comparison to growth in vitro. Mar Biol 75:293–302
Strom SL (1991) Growth and grazing rates of the herbivorous dinoflagellate Gymnodinium sp. from the open subarctic Pacific Ocean. Mar Ecol Prog Ser 78:103–113
Strom SL, Welchmeyer NA (1991) Pigment-specific rates of phytoplankton growth and microzooplankton grazing in the open subarctic Pacific Ocean. Limnol Oceanogr 36:50–63
Thomsen HA (1986) A survey of the smallest eukaryotic organisms of the marine phytoplankton. In: Platt T, Li WKW (eds) Photosynthetic picoplankton. Can Bull Fish Aquat Sci 214:121–130
Valout D, Partensky F (1992) Cell cycle distribution of prochlorophytes in the northwestern] Mediterranean Sea. Deep-Sea Res 39:727–742
Veldhuis MJW, Kraay GW (1990) Vertical distribution and pigment composition of a picoplanktonic prochlorophyte in the subtrophical North Atlantic: a combined study of HPLC-analysis of pigments and flow cytometry. Mar Ecol Prog Ser 68:121–127
Verity PG (1985) Grazing, respiration, excretion, and growth rates of tintinnids. Limnol Oceanogr 30:1268–1281
Verity PG (1991) Measurement and simulation of prey uptake by marine planktonic ciliates fed plastidic and aplastidic nanoplankton. Limnol Oceanogr 36:729–750
Verity PG, Stoecker DK, Sieracki ME, Burkill PH, Edwards ES, Tronzo CR (1993) Abundance, biomass, and distribution of heterotrophic dinoflagellates during the North Atlantic Spring Bloom. Deep-Sea Res 40:227–244
Waterbury JB, Watson SW, Guillard RRL, Brand LE (1979) Widespread occurrence of a unicellular, marine, planktonic cyanobacterium. Nature 227:293–294
Waterbury JB, Watson SW, Valois FW, Franks DG (1986) Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. In: Platt T, Li WKW (eds) Photosynthetic picoplankton. Can Bull Fish Aquat Sci 214:71–120
Williams PJLeB (1981) Incorporation of microheterotrophic processes into the classical paradigm of the planktonic food web. Kieler Meerresforsch Sonderh 5:1–28
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sherr, E.B., Sherr, B.F. Bacterivory and herbivory: Key roles of phagotrophic protists in pelagic food webs. Microb Ecol 28, 223–235 (1994). https://doi.org/10.1007/BF00166812
Issue Date:
DOI: https://doi.org/10.1007/BF00166812