Summary
Conventional solution-phase polymerase chain reaction (PCR) and in situ PCR/PCR in situ hybridization are powerful tools for retrospective analysis of fixed paraffin wax-embedded material. Amplification failure using these techniques is now encountered in some centres using archival fixed tissues. Such ◂ailures’ may not only be due to absent target DNA sequences in the tissues, but may be a direct effect of the type of fixative, fixation time and/or fixation temperature used. The type of nucleic acid extraction procedure applied will also influence amplification results. This is particularly true with in situ PCR/PCR in situ hybridization.
To examine these effects in solution-phase PCR, β-globin gene was amplified in 100 mg pieces of tonsillar tissue fixed in Formal saline, 10% formalin, neutral buffered formaldehyde, Carnoy's, Bouin's, buffered formaldehyde sublimate, Zenker's, Helly's and glutaraldehyde at 0 to 4°C, room temperature and 37°C fixation temperatures and for fixation periods of 6, 24, 48 and 72 hours and 1 week. DNA extraction procedures used were simple boiling and 5 days' proteinase K digestion at 37°C. Amplified product was visible primarily yet variably from tissue fixed in neutral buffered formaldehyde and Carnoy's, whereas fixation in mercuric chloride-based fixatives produced consistently negative results. Room temperature and 37°C fixation temperature appeared most conducive to yielding amplifiable DNA template. Fixation times of 24 and 48 hours in neutral buffered formaldehyde and Carnoy's again favoured amplification.
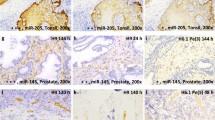
Fixed SiHa cells (containing 1–2 copies of HPV 16) were examined using PCR in situ hybridization for the amplification of HPV 16. Discrete and diffuse amplification signals were obtained. Neutral buffered formaldehyde fixation for 12–24 hours yielded amplifiable material suitable for use with PCR in situ hybridization. Overall amplification success within cellular preparations was 40%, with non-specific background staining also seen. Possible technical problems encountered with PCR in situ hybridization are discussed.
Similar content being viewed by others
References
An, S. F. & Fleming, K. A. (1991) Removal of inhibitor(s) of the polymerase chain reaction from formalin fixed, paraffin wax embedded tissues. J. Clin. Pathol. 44, 924–7.
Barton Rogers, B., Alpert, L. C., Hine, E. A. S. & Buffone, G. J. (1990) Analysis of DNA in fresh and fixed tissue by the polymerase chain reaction. Am. J. Pathol. 136, 541–8.
Battifora, H. & Kopinski, M. (1986) The influence of proteinase digestion and duration of fixation on the immunostaining of keratins. A comparison of formalin and ethanol fixation. J. Histochem. Cytochem. 34, 1095.
Doyle, C. T. & O'Leary, J. J. (1992) The search for the universal fixative or ‘magic juice’. J. Pathol. 166, 331–2.
Dubeau, L., Chandler, L. A., Gralow, J. R., Nichols, P. W. & Jones, P. A. (1986) Southern blot analysis of DNA extracted from formalin fixed pathology specimens. Cancer Res. 46, 2964–9.
Goelz, S. E., Hamilton, S. R. & Vogelstein, B. (1985) Purification of DNA from formaldehyde fixed and paraffin embedded human tissue. Biochem. Biol. Res. Commun. 130, 118–26.
Greer, C. E., Patterson, S. L., Kiviat, N. B. & Manos, M. (1991) PCR amplification from paraffin embedded tissues; effects of fixative and fixation time. Am. Clin. Pathol. 95, 117–24.
Hopwood, D. (1972) Theoretical and practical aspects of glutaraldehyde fixation. Histochem. J. 4, 267–303.
Hopwood, D. (1985) Cell and tissue fixation, 1972–1982. Histochem. J. 17, 389–442.
Jackson, D. P., Lewis, F. A., Taylor, G. R., Boylston, A. W. & Quirke, P. (1990) Tissue extraction of DNA and RNA and analysis by the polymerase chain reaction. J. Clin. Pathol. 43, 499–504.
Lench, N., Stanier, P. & Williamson, R. (1988) Simple noninvasive method to obtain DNA for gene analysis. Lancet ii, 1356–8.
Lewis, F. A., Griffiths, S., Dunnicliff, R., WellsM., Dudding, N. & BirdC. C. (1987) Sensitive in-situ hybridisation technique using biotin-streptavidin polyalkaline phosphatase complex. J. Clin. Pathol. 40, 163–66.
Mcghee, J. D. & VonHippel, P. H. (1975a) Formaldehyde as a probe of DNA structure 1. Reaction with exocyclic amino groups of DNA bases. Biochemistry 14, 1281–95.
Mcghee, J. D. & VonHippel, P. H. (1975b) Formaldehyde as a probe of DNA structure 2. Reaction with endocyclic imino groups of DNA bases. Biochemistry 14, 1297–303.
Mcghee, J. D. & VonHippel, P. H. (1976a) Formaldehyde as a probe of DNA structure 3. Mechanism of the initial reaction of formaldehyde with DNA. Biochemistry 16, 3267–76.
Mcghee, J. D. & VonHippel, P. H. (1976b) Formaldehyde as a probe of DNA structure 4. Equilibrium denaturation of DNA and synthetic polynucleotides. Biochemistry 16, 3276–93.
Nuovo, G. J., Gallery, F., Macconnell, P., Becker, P. & Bloch, W. (1991) An improved technique for the detection of DNA by in-situ hybridisation after PCR amplification. Am. J. Pathol. 139, 1239–44.
Nuovo, G. J. (1992) PCR in situ hybridization. Protocols and Applications. New York: Raven Press.
Nuovo, G. J., Gallery, F., Hom, R., Macconnell, P. & Bloch, W. (1993) Importance of different variables for enhancing in-situ detection of PCR amplified DNA. PCR Meth. App. 305–12.
O'Leary, J. J., Browne, G., Bashir, M. S., Crowley, M., HEALY, I., Lewis, F. A. & Doyle, C. T. (1994) Non-Isotopic Detection of DNA in tissues. Practical approach series. Oxford: Oxford University Press (in press).
Saiki, R. H., Scharf, S., Faloona, F., Mullis, K. B., Horn, G. T., Erlich, H. A., & Arnheim, N. (1985) Enzymatic amplification of β-globin genetic sequence and restriction site analysis for diagnosis of sickle cell anaemia. Science 230, 1350–4.
Shibata, D., Forrester, K., Martin, J., Arnheim, N. & PeruchoM. (1988) Most human carcinomas of exocrine pancreas contain mutant K-ras genes. Cell 53, 549.
Sheibani, K., Wu, A., Ben-Ezra, J. & Battifora, H. (1989) Analysis of Ha-Ras sequence in DNA's of malignant mesothelioma and pulmonary adenocarcinoma by a sensitive polymerase chain reaction (PCR) method. Lab. Invest. 60, 87A.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
O'Leary, J.J., Browne, G., Landers, R.J. et al. The importance of fixation procedures on DNA template and its suitability for solution-phase polymerase chain reaction and PCR in situ hybridization. Histochem J 26, 337–346 (1994). https://doi.org/10.1007/BF00157767
Received:
Revised:
Issue Date:
DOI: https://doi.org/10.1007/BF00157767