Abstract
Soil seed banks and current seed inputs each play a role in tropical succession. We compared the abundance and floristic composition of seeds from these two sources at a Costa Rican site by germinating seeds from the soil, measuring seed inputs for 3 yr, and monitoring the earliest colonists in a forest clearing.
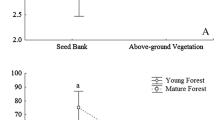
There were an estimated 6800 viable seeds/m2 in the soil of 3.3-yr-old vegetation, 9500 seeds/m2 in 11-yr-old vegetation, and 7000 seeds/m2 in a 75-yr-old forest. An estimated 10100 seeds/m2 fell on the soil surface of the young successional vegetation during 3 yr and 3700 seeds/m2 fell during that same time in the forest.
Locally produced seeds accounted for about 75% of the seed input to the soil surface early in succession. Seeds dispersed out of young successional vegetation increased the quantity and species richness of the seed input and storage in an adjacent forest. Much of the species richness of the young successional vegetation resulted from seeds dispersed there from other communities by animals.
Deforestation stimulated germination of most seeds in the surface soil of the old forest, including seeds of the dominant canopy tree. The recruitment of seedlings from the soil seed bank numerically overwhelmed that from post-disturbance seed rain and sprouts.
We evaluated patterns of soil seed storage during succession and predicted the ability of vegetation of differing ages to respond to disturbance. Immediately after disturbance the number of seeds in the soil plummeted due to mortality, low inputs, and germination. As the vegetation regrew, the soil seed bank increased to a peak after 4 to 7 yr, then gradually decreased to its pre-disturbance size. High-frequency pulses of disturbance should result in reduced species richness, dominance by species with long-lived seeds, and fast recovery by seedling recruitment from the soil seed bank.
Similar content being viewed by others
References
Ashton P. S. 1978. Crown characteristics of tropical trees. In: Tomlinson P. B. & Zimmerman M. H. (eds), Tropical trees as living systems, pp. 591–615. Cambridge Univ. Press, Cambridge.
Bell C. R. 1970. Seed distribution and germination experiment. In: Odum H. T. & Pigeon R. F. (eds), A tropical rain forest, a study of irradiation and ecology at El Verde, Puerto Rico, pp. D177-D182. U. S. Atomic Energy Comm., Oak Ridge, Tennessee.
Berish C. W. 1983. Roots, soil, litter, and nutrient changes in simple and diverse tropical successional ecosystems. Ph.D. Dissertation. Univ. of Florida, Gainesville.
Blanton C. M. & Ewel J. J. 1985. Leaf-cutting ant herbivory in successional and agricultural tropical ecosystems. Ecology 66: 861–869.
Brokaw N. V. L. 1980. Gap-phase regeneration in a neotropical forest. Ph. D. Dissertation. University of Chicago, Chicago, Illinois.
Carabias-Lilo J. & Guevara S. 1985. Fenología en una selva tropical húmeda y en una comunidad derivada; Los Tuxtlas, Veracruz. In: Gómez-Pompa A. & del Amo R. S. (eds), Investigaciones sobre la regeneración de selvas altas en Veracruz, Mexico. Vol. II, pp. 27–66. Instituto Nacional de Investigaciones sobre Recursos Bioticos, Xalapa, Veracruz, Mexico.
Cheke A. S., Nanakorn W. & Yankoses C. 1979. Dormancy and dispersal of seeds of secondary forest species under the canopy of a primary tropical rain forest in northern Thailand. Biotropica 11: 88–95.
Estrada Pinto A. 1970. Phenological studies of trees of El Verde. In: Odum H. T. & Pigeon R. F. (eds), A tropical rain forest, A study of irradiation and ecology at El Verde, Puerto Rico, pp. D237-D269. U.S. Atomic Energy Comm., Oak Ridge, Tennessee.
Ewel J. 1977. Differences between wet and dry successional tropical ecosystems. Geo-Eco-Trop 1: 103–117.
Ewel J., Gliessman S., Amador M., Benedict F., Berish C., Bermúdez R., Brown B., Martínez A., Miranda R. & Price N. 1982. Leaf area, light transmission, roots and leaf damage in nine tropical plant communities. Agro-Ecosystems 7: 305–326.
Ewel J., Berish C., Brown B., Price N. & Raich J. 1981. Slash and burn impacts on a Costa Rican wet forest site. Ecology 62: 816–829.
Foster R. B. 1982. The seasonal rhythm of fruitfall on Barro Colorado Island. In: LeighJr E. G., Rand A. S. & Winsor D. M. (eds), The ecology of a tropical forest: seasonal rhythms and long-term changes, pp. 151–172. Smithsonian Institution Press, Washington, D.C.
Garwood N. C. 1983. Seed germination in a seasonal tropical forest in Panama: a community study. Ecol. Monogr. 53: 159–181.
Gómez-Pompa A., Vázquez-Yanes C. & Guevara S. 1972. The tropical rain forest: a nonrenewable resource. Science 177: 762–765.
Gross K. L. & Werner P. A. 1982. Colonizing abilities of ‘biennial’ plant species in relation to ground cover: implications for their distributions in a successional sere. Ecology 63: 921–931.
Grubb P. J. 1977. The maintenance of species-richness in plant communities: the importance of the regeneration niche. Biol. Rev. 52: 107–145.
Guevara, S. A. 1986. Plant species availability and regeneration in a Mexican tropical rain forest. Ph.D. Dissertation. Uppsala University.
Guevara S. & Gómez-Pompa A. 1972. Seeds from surface soils in a tropical region of Veracruz, Mexico. J. Arnold Arboretum 53: 312–335.
Hall J. B. & Swaine M. D. 1980. Seed stocks in Ghanian forest soils. Biotropica 12: 256–263.
Harcombe P.A. 1977. The influence of fertilization on some aspects of succession in a humid tropical forest. Ecology 58: 1375–1383.
Harper J. L. 1977. Population biology of plants. Academic Press, New York.
Hopkins B. 1970. Vegetation of the Olokemeji Forest Reserve, Nigeria. VI. The plants of the forest site with special reference to their seasonal growth. J. Ecol. 58: 765–793.
Hopkins M. S. & Graham A. W. 1983. The species composition of soil seed banks beneath lowland tropical rainforests in North Queensland, Australia. Biotropica 15: 90–99.
Jackson J. F. 1981. Seed size as a correlate of temporal and spatial patterns of seed fall in neotropical forest. Biotropica 13: 121–130.
Janzen D. H. 1983. No park is an island: increase in interference from outside as park size decreases. Oikos 41: 402–410.
Keay, R. W. J. 1960. Seeds in forest soil. Nigerian Forestry Information Bull. N.S. 4.
Kellman M. C. 1974. Preliminary seed budgets for two plant communities in coastal British Columbia. J. Biogeogr. 1: 123–133.
Kellman M. C. 1978. Microdistribution of viable weed seed in two tropical soils. J. Biogeogr. 5: 291–300.
Liew T. C. 1973. Occurrence of seeds in virgin forest top soil with particular reference to secondary species in Sabah. Malaysian Forester 36: 185–193.
Livingston R. B. & Allessio M. 1968. Buried viable seed in successional field and forest stands, Harvard Forest, Mass. Bull. Torrey Bot. Club 95: 58–69.
Marks P. L. 1983. On the origin of the field plants of the northeastern United States. Amer. Nat. 122: 210–228.
Noble I. R. & Slatyer R. O. 1980. The use of vital attributes to predict successional changes in plant communities subject to recurrent disturbances. Vegetatio 43: 5–21.
Putz F. E. 1983. Treefall pits and mounds, buried seeds, and the importance of soil disturbance to pioneer trees on Barro Colorado Island, Panama. Ecology 64: 1069–1074.
Rampton H. A. & Ching T. M. 1970. Persistance of crop seeds in soils. Agron. J. 62: 272–277.
Roberts H. A. 1981. Seed banks in soils. Adv. Applied Biol. 6: 1–55.
Saulei S. M. 1984. Natural regeneration following clear-fell logging operations in the Gogol Valley, Papua New Guinea. Ambio 13: 351–354.
Smythe N. 1970. Relationships between fruiting seasons and seed dispersal methods in a neotropical forest. Amer. Nat. 104: 25–35.
Swaine M. D. & Hall J. B. 1983. Early succession on cleared forest land in Ghana. J. Ecol. 71: 601–627.
Sydes C., & Grime J. P. 1981. Effects of tree leaf litter on herbaceous vegetation in deciduous woodland. II. An experimental investigation. J. Ecol. 69: 249–262.
Thompson K., Grime J. P. & Mason G. 1977. Seed germination in response to diurnal fluctuations of temperature. Nature 267: 147–149.
TosiJr. J. A. 1969. Mapa ecológico de Costa Rica. Centro Científico Tropical, San José, Costa Rica.
Uhl C. 1982. Recovery following disturbances of different intensities in the Amazon rain forest of Venezuela. Interciencia 7: 19–24.
Uhl C., Clark H., Clark K. & Maquirino P. 1982. Successional patterns associated with slash-and-burn agriculture in the upper Rio Negro region of the Amazon Basin. Biotropica 14: 249–254.
Uhl C. & Clark K. 1983. Seed ecology of selected Amazon basin successional species. Bot. Gaz. 144: 419–425.
Uhl C., Clark K., Clark H. & Murphy P. 1981. Early plant succession after cutting and burning in the upper Rio Negro of the Amazon Basin. J. Ecol. 69: 631–649.
Vazques-Yanes C. 1976. Seed dormancy and germination in secondary vegetation tropical plants: the role of light. Comparative Physiological Ecol. 1: 30–34.
Van der Valk A. G. & Davis C. B. 1978. The role of seed banks in the vegetation dynamics of prairie glacial marshes. Ecology 59: 332–335.
Whitmore T. C. 1982. On pattern and process in forest. In: Newman E. I. (ed.), The plant community as a working mechanism, pp. 45–59. Brit. Ecol. Soc. Blackwell, Oxford.
Whitmore T. C. 1983. Secondary succession from seed in tropical rain forests. Commonwealth Forestry Bur., For. Abstr. 44: 767–779.
Williams-Linera G. & Ewel J. J. 1984. Effect of autoclave sterilization of a tropical andept on seed germination and seedling growth. Plant & Soil 82: 263–268.
Young K. R. 1985. Deeply buried seeds in a tropical wet forest in Costa Rica. Biotropica 17: 336–338.
Author information
Authors and Affiliations
Additional information
Journal series number 6459 from the Institute of Food and Agricultural Sciences, University of Florida, Gainesville, Florida 32611, USA.
Reprint requests to J. J. E. at Florida.
Rights and permissions
About this article
Cite this article
Young, K.R., Ewel, J.J. & Brown, B.J. Seed dynamics during forest succession in Costa Rica. Vegetatio 71, 157–173 (1987). https://doi.org/10.1007/BF00039168
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00039168