Abstract
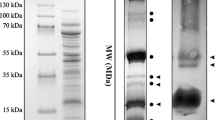
A recent report (Nanba O, Satoh K: Proc. Natl. Acad. Sci. USA 84: 109–112, 1987) described the isolation from spinach of a putative photosystem 2 reaction centre which contained cytochrome b-559 and three other electrophoretically resolvable polypeptide bands, two of which have molecular weights comparable to the D1 and D2 polypeptides. We have used in vivo labelling with radioactive methionine and probed with D1 and D2 monospecific antibodies (raised against synthetically expressed sequences of the psbA and psbD genes) for specific detection of these proteins in a similarly prepared photosystem 2 reaction centre preparation. These techniques identified a 32 000 dalton D1 band, a 30 000 dalton D2 band and a 55 000 dalton D1/D2 aggregate, the latter apparently arising from the detergent treatments employed. Digestions with a lysine-specific protease further confirmed the identification of the lysine-free D1 polypeptide and also confirmed that the D1 molecules in the 55 000 dalton band were in aggregation with other bands and not in self-aggregates. The D1 and D2 polypeptides (including the aggregate) are considerably enriched in the photosystem two reaction centre preparation compared to the other resolved fractions.
Similar content being viewed by others
References
Barber J, Marder JB: Photosynthesis and the application of molecular genetics. In: Russell M (ed.) Biotechnology and Genetic Engineering Reviews, Vol. 4, pp. 355–404. Newcastle-upon-Tyne: Intercept (1986).
Berthold DA, Babcock GT, Yocum CJ: A highly resolved, oxygen-evolving photosystem II preparation from spinach thylakoid membranes. FEBS Lett 134: 231–234 (1981).
Bonner WM, Laskey RA: A film detection method for tritium labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem 76: 83–88 (1974).
Breton J: The 695 nm fluorescence (F695) of chloroplasts at low temperature is emitted from the primary acceptor of photosystem II. FEBS Lett 147: 16–20 (1982).
Bricker TM, Frankel LK: Characterization of a monoclonal antibody which reacts with the 49 kDa polypeptide of Photosystem II from spinach. In: Biggins J (ed.) Progress in Photosynthesis Research, Vol. 2, pp. 129–132. Dordrecht: Martinus Nijhoff (1987).
Chang C-H, Tiede D, Tang J, Smith U, Norris J, Schniffer M: Structure of Rhodopseudomonas sphaeroides R-26 reaction centre. FEBS Lett 205: 82–86 (1986).
Chua N-H, Gillham NW: The sites of synthesis of principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. Cell Biol 74: 441–452 (1977).
Deisenhofer J, Epp O, Miki K, Huber R, Michel H: Structure of the protein subunits in the photosynthetic reaction centre of Rhodopseudomonas viridis at 3A resolution. Nature 318: 618–624 (1985).
Dunn SD: Effects of the modification of transfer buffer composition and the renaturation of proteins in gels on the recognition of proteins on Western blots by monoclonal antibodies. Analytical Biochem 157: 144–153 (1986).
Eaglesham ARJ, Ellis RJ: Protein synthesis in chloroplasts II: light-driven synthesis of membrane proteins by isolated pea chloroplasts. Biochim Biophys Acta 335: 396–407 (1974).
Gounaris K, Pick U, Barber J: Stoichiometry and turnover of photosystem two polypeptides. FEBS Lett 211: 94–98 (1987).
Hearst JE, Sauer K: Protein sequence homologies between positions of the L and M subunits of reaction centres of Rhodopseudomonas capsulata and the QB-protein of chloroplast thylakoid membranes: a proposed relation to quinone-binding sites. Z Naturforsch 39c: 421–424 (1984).
Herrmann RG, Alt J, Schiller B, Widger WR, Cramer WA: Nucleotide sequence of the gene for apocytochrome b-559 on the spinach plastid chromosome. FEBS Lett 176: 239–244 (1984).
Kyle DJ: The 32 000 dalton QB protein of photosystem II. Photobiochem Photobiol 41: 107–116 (1985).
Laemmli UK: Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227: 680–685 (1970).
Marder JB, Mattoo AR, Edelman M: Identification and characterization of the psbA gene product: The 32 kDa chloroplast membrane protein. Methods in Enzymol 188: 384–396 (1986).
Murata N, Miyao M: Extrinsic membrane proteins in the photosynthetic oxygen-evolving complex. Trends Biochem Sci 10: 122–124 (1985).
Nakatani HY, Ke B, Dolan E, Arntzen CJ: Identity of the photosystem II reaction centre polypeptide. Biochim Biophys Acta 756: 347–352 (1984).
Nanba O, Satoh K: Isolation of a photosystem II reaction center containing D-1 and D-2 polypeptides and cytochrome b-559. Proc Natl Acad Sci USA 84: 109–112 (1987).
Nixon PJ, Dyer T, Barber J, Hunter CN: Immunological evidence for the presence of the D1 and D2 proteins in PSII cores of higher plants. FEBS Lett 209: 83–86 (1987).
Okamura MY, Satoh K, Isaacson RA, Feher G: Evidence of the primary change separation in the D1D2 complex of photosystem II from spinach: EPR of the triplet state. In: Biggins J (ed.) Progress in Photosynthesis Research, Vol. 1, pp. 379–381, Dordrecht: Martinus Nijhoff (1987).
Rochaix J-D, Dron M, Rahire M, Malnoe P: Sequence homology between the 32 K dalton and the D2 chloroplast membrane polypeptides of Chlamydomonas reinhardtii. Plant Mol Biol 3: 363–370 (1984).
Rutherford AW: How close is the analogy between PSII and purple bacteria. Biochem Soc Trans, Oxford 14: 15–17 (1986).
Satoh K, Nakatani HY, Steinback KE, Watson J, Arntzen CJ: Polypeptide composition of a photosystem II core complex; presence of a herbicide-binding protein. Biochim Biophys Acta 724: 142–150 (1983).
Satoh K, Nanba O: Isolation of a photosystem II reaction center consisting of gamma and delta subunits (D-1 and D-2) and cytochrome b-559. In: Biggins J (ed.) Progress in Photosynthesis Research, Vol. 2, pp. 69–72. Dordrecht: Martinus Nijhoff (1987).
Trebst A: The topology of the plastoquinone and herbicide binding peptides of photosystem II in the thylakoid membrane. Z Naturforsch 41c: 240–245 (1985).
Williams JC, Steiner LA, Feher G, Simon MJ: Primary structure of the L subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci USA 81: 7303–7301 (1984).
Zurawski G, Bohnert HJ, Whitfeld PR, Bottomley W: Nucleotide sequence of the gene for the 32 000-Mr thylakoid membrane protein from Spinacia oleracea and Nicotiana debneyii predicts a totally conserved primary translation product of Mr 38800. Proc Natl Acad Sci USA 79: 7699–7703 (1982).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Marder, J.B., Chapman, D.J., Telfer, A. et al. Identification of psbA and psbD gene products, D1 and D2, as reaction centre proteins of photosystem 2. Plant Mol Biol 9, 325–333 (1987). https://doi.org/10.1007/BF00014907
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00014907