Abstract
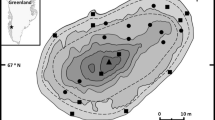
The abundance and distribution of dissolved CH4 were determined from 1987–1990 in Lake Fryxell, Antarctica, an amictic, permanently ice-covered lake in which solute movement is controlled by diffusion. CH4 concentrations were < 1 υM in the upper oxic waters, but increased below the oxycline to 936 μM at 18 m. Sediment CH4 was 1100 μmol (1 sed)−1 in the 0–5 cm zone. Upward flux from the sediment was the source of the CH4, NH4 +, and DOC in the water column; CH4 was 27% of the DOC+CH4 carbon at 18 m. Incubations with surficial sediments indicated that H14CO3 − reduction was 0.4 μmol (1 sed)−1 day−1 or 4× the rate of acetate fermentation to CH4. There was no measurable CH4 production in the water column. However, depth profiles of CH4, NH4, and DIC normalized to bottom water concentrations demonstrated that a significant CH4 sink was evident in the anoxic, sulfate-containing zone of the water column (10–18 m). The δ13CH4 in this zone decreased from −72 % at 18 m to −76% at 12 m, indicating that the consumption mechanism did not result in an isotopic enrichment of 13CH4. In contrast, δ13CH4 increased to −55 % at 9 m due to aerobic oxidation, though this was a minor aspect of the CH4 cycle. The water column CH4 profile was modeled by coupling diffusive flux with a first order consumption term; the best-fit rate constant for anaerobic CH4 consumption was 0.012 yr−1. On a total carbon basis, CH4 consumption in the anoxic water column exerted a major effect on the flux of carbonaceous material from the underlying sediments and serves to exemplify the importance of CH4 to carbon cycling in Lake Fryxell.
Similar content being viewed by others
References
Aiken GR (1991) The nature of nonvolatile organic acids in antarctic lakes. Ph.D. Dissertation, Colorado School of Mines
Aiken GR, McKnight DM, Wershaw R & Miller L (1991) Evidence for the diffusion of aquatic fulvic acid from the sediments of Lake Fryxell, Antarctica. In: Baker RA (Ed) Organic Substances and Sediments in Water. Vol. 1. Humics and Soils (pp 75–88). Lewis Publishers, Chelsea, MI
Alperin MJ, Reeburgh WS & Whiticar MJ (1988) Carbon and hydrogen isotope fractionation resulting from anaerobic methane oxidation. Global Biogeochem. Cycles 2: 279–288
Berner RA (1980) Early Diagenesis: A Theoretical Approach. Princeton University Press, Princeton, NJ
Burton HR (1980) Methane in a saline antarctic lake. In: Trudinger PA & Walter MR (Ed) Biogeochemistry of Ancient and Modern Environments (pp 243–251). Springer-Verlag, New York, NY
Burton HR (1981) Chemistry, physics, and evolution of Antarctic saline lakes. Hydrobiol. 82: 339–362
Claypool GE & Kaplan IR (1974) The origin and distribution of methane in marine sediments. In: Kaplan IR (Ed) Natural Gases In Marine Sediments (pp 99–139). Plenum Inc., New York, NY
Claypool GE & Kvenvolden KA (1983) Methane and other hydrocarbon gases in marine sediment. Ann. Rev. Earth and Planet. Sci. 11: 299–327
Coleman DD, Risatti JB & Schoell M (1981) Fractionation of carbon and hydrogen isotopes by methane-oxidizing bacteria. Geochim. Cosmochim. Acta 45: 1033–1037
Craig H, Wharton RA & McKay CP (1992) Oxygen supersaturation in ice-covered antarctic lakes: Biological versus physical contributions. Science 255: 318–321
Culbertson CW, Zehnder AJ & Oremland RS (1981) Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl. Environ. Microbiol. 41: 396–403
Downes MT, Howard-Williams C & Vincent WF (1986) Sources of organic nitrogen, phosphorous and carbon in antarctic streams. Hydrobiol. 134: 215–225
Ellis-Evans JC (1984) Methane in maritime antarctic freshwater lakes. Polar Biol. 3: 63–71
Franzmann PD, Roberts NJ, Mancuso CA, Burton HR & McMeekin TA (1991) Methane production in meromictic Ace Lake, Antarctica. Hydrobiol. 210: 191–201
Green TGA (1985) Dry valley terrestrial plant communities: The problem of production and growth. New Zealand Antarctic Record 6: 40–44
Green WJ, Gardner TJ, Ferdelman TG, Angle MP, Varner LC & Nixon P (1989) Geochemical processes in the Lake Fryxell basin (Victoria Land, Antarctica). Hydrobiol. 172: 129–148
Gunsalus RP, Romesser JA & Wolfe RS (1978) Preparation of coenzyme M analogues and their activity in the methyl coenzyme M reductase system of Methanobacterium thermoautotrophicum. Biochem. 17: 2374–2377
Howard-Williams C & Vincent WF (1989) Microbial communities in southern Victoria Land streams (Antarctica) I. Photosynthesis. Hydrobiol. 172: 27–38
Howes BL & Smith RL (1990) Sulfur cycling in a permanently ice covered amictic antarctic lake, Lake Fryxell. Ant. J. US 25: 230–233
Ingvorsen K, Zeikus JG & Brock TD (1981) Dynamics of bacterial sulfate reduction in a eutrophic lake. Appl. Environ. Microbiol. 42: 1019–1036
Iversen N & Jörgensen BB (1985) Anaerobic methane oxidation rates at the sulfatemethane transition in marine sediments from Kattegat and Skagerrak (Denmark). Limnol. Oceanogr. 30: 944–955
Iversen N, Oremland RS & Klug MJ (1987) Big Soda Lake (Nevada). 3. Pelagic methanogenesis and anaerobic methane oxidation. Limnol. Oceanogr. 32: 804–814
Javor B (1989) Hypersaline Environments. Springer-Verlag, New York, NY
King GM (1990) Regulation by light of methane emissions from a wetland. Nature 345: 513–515
Kuivila KM, Murray JW, Devol AH, Lidstrom ME & Reimers CE (1988) Methane cycling in the sediments of Lake Washington. Limnol. Oceanogr. 33: 571–581
Lawrence MJF & Hendy CH (1985) Water column and sediment characteristics of Lake Fryxell, Taylor Valley, Antarctica. New Zealand J. Geol. Geophys. 28: 543–552
Lawrence MJF & Hendy CH (1989) Carbonate deposition and Ross Sea ice advance, Fryxell basin, Taylor Valley, Antarctica. New Zealand J. Geol. Geophys. 32: 267–277
Lerman A (1979) Geochemical Processes: Water and Sediment Environments. John Wiley & Sons, New York, NY
Li Y & Gregory S (1974) Diffusion of ions in sea water and in deep–sea sediments. Geochim. Cosmochim. Acta 38: 703–714
Martens CS & Berner RA (1977) Interstitial water chemistry of anoxic Long Island Sound sediments. I. Dissolved gases. Limnol. Oceanogr. 22: 10–25
Martens CS, Blair NE, Green CD & DesMarais DJ (1986) Seasonal variations in the stable carbon isotope signature of biogenic methane in a coastal sediment. Science 233: 1300–1303
Matsubaya O, Sakai H, Torii T, Burton H & Kerry K (1979) Antarctic saline lakes-stable isotopic ratios, chemical compositions and evolution. Geochim. Cosmochim. Acta 43: 7–25
Matsumoto GI (1989) Biogeochemical study of organic substances in antarctic lakes. Hydrobiol. 172: 265–289
Matsumoto GI, Watanuki K & Torii T (1989) Vertical distribution of organic constituents in an antarctic lake: Lake Fryxell. Hydrobiol. 172: 291–303
McKnight DM, Aiken GR & Smith RL (1991) Aquatic fulvic acids in microbially-based ecosystems: Results from two desert lakes in Antarctica. Limnol. Oceanogr. 36: 998–1006
Molongoski JJ & Klug MJ (1980) Anaerobic metabolism of particulate organic matter in the sediments of a hypereutrophic lake. Freshwat. Biol. 10: 507–518
Oremland RS & DesMarais DJ (1983) Distribution, abundance, and carbon isotopic composition of gaseous hydrocarbons in Big Soda Lake, Nevada: An alkaline, meromictic lake. Geochim. Cosmochim. Acta 47: 2107–2114
Oremland RS, Miller LG & Whiticar MJ (1987) Sources and flux of natural gases from Mono Lake, California. Geochim. Cosmochim. Acta 51: 2915–2929
Priscu JC, Priscu LR, Vincent WF & Howard-Williams C (1987) Photosynthate distribution by microplankton in permanently ice-covered antarctic desert lakes. Limnol. Oceanogr. 32: 260–270
Priscu JC, Vincent WF & Howard-Williams C (1988) Inorganic nitrogen uptake and regeneration in perennially ice-covered Lakes Fryxell and Vanda, Antarctica. J. Plank. Res. 11: 335–351
Reeburgh WS (1980) Anaerobic methane oxidation: Rate depth distributions in Skan Bay sediments. Earth Planet. Sci. Lett. 47: 345–352
Reeburgh WS, Ward BB, Whalen SC, Sandbeck KA, Kilpatrick KA & Kerkhof LJ, (1991) Black Sea methane geochemistry. Deep Sea Res. 38: S1189–S1210
Rudd JWH & Hamilton RD (1978) Methane cycling in a eutrophic shield lake and its effects on whole lake metabolism. Limnol Oceanogr. 23: 337–348
Scheiner D (1976) Determination of ammonia and Kjeldahl nitrogen by the indophenol method. Water Res. 10: 31–36
Smith RL & Howes BL (1990) Bacterial biomass and heterotrophic activity in the water column of an amictic antarctic lake. Ant. J. US 25: 233–235
Smith RL & Klug MJ (1981) Reduction of sulfur compounds in the sediments of a eutrophic lake basin. Appl. Environ. Microbiol. 41: 1230–1237
Smith RL & Klug MJ (1987) Flowthrough reactor flasks for study of microbial metabolism in sediments. Appl. Environ. Microbiol. 53: 371–374
Tabatabai MA (1974) Determination of sulfate in water samples. Sulphur Inst. J. 10: 11–13
Torii T & Yamagata N (1981) Limnological studies of saline lakes in the Dry Valleys. In: McGinnis LD (Ed) Dry Valley Project (pp 141–159). Amer. Geophys. Union, Washington, DC
Torii T, Yamagata N, Nakaya S, Murata S, Hashimoto T, Matsubaya O & Sakai H (1975) Geochemical aspects of the McMurdo saline lakes with special emphasis on the distribution of nutrient matters. In: Torii T (Ed) Geochemical and Geophysical Studies of Dry Valleys, Victoria Land in Antarctica (pp 5–29). National Institute of Polar Research Tokyo, Japan
Vincent WF (1981) Production strategies in antarctic inland waters: Phytoplankton ecophysiology in a permanently ice-covered lake. Ecol. 62: 1215–1224
Waguri O (1976) Isolation of microorganisms from salt lakes in the Dry Valley, Antarctica, and their living environment. Antarct. Rec. 57: 80–96
Ward BB, Kilpatrick KA, Novelli PC & Scranton MI (1987) Methane oxidation and methane fluxes in the ocean surface layer and deep anoxic waters. Nature 327: 226–229
Whalen JK, Oremland R, Tarafa M, Smith R, Howarth R & Lee C (1986) Evidence for sulfate-reducing and methane-producing microorganisms in sediments from sites 618, 619, and 622. In: Bouma AH, Coleman JM, Meyer AW et al.(Eds) Init. Repts. Deep Sea Drill. Prog., 96(pp 767–775). US Govt. Printing Office, Wasington, DC
Wharton RA, McKay CP, Mancinelli RL & Simmons GM (1987) Perennial N2 supersaturation in an antarctic lake. Nature 325: 343–345
Wharton RA, McKay CP, Simmons GM & Parker BC (1986) Oxygen budget of a perennially ice-covered antarctic lake. Limnol. Oceanogr. 31: 437–443
Wiesenburg DA, Brooks JM & Bernard BB (1985) Biogenic hydrocarbon gases and sulfate reduction in the Orca Basin brine. Geochim. Cosmochim. Acta 49: 2069–2080
Wilson AT (1964) Evidence from chemical diffusion of a climatic change in the McMurdo Dry Valleys 1,200 years ago. Nature 201: 176–177
Winfrey MR, Nelson DR, Klevickis SC & Zeikus JG (1977) Association of hydrogen metabolism with methanogenesis in Lake Mendota sediments. Appl. Environ. Microbiol. 33: 312–318
Winfrey MR & Zeikus JG (1979) Microbial methanogenesis and acetate metabolism in a meromictic lake. Appl. Environ. Microbiol. 37: 213–221
Yarrington MR & Wynn-Williams DD (1985) Methanogenesis and the anaerobic microbiology of a wet moss community at Signy Island. In: Siefried WR, Condy PR & Laws RM (Eds) Antarctic Nutrient Cycles and Food Webs (pp 229–233). Springer-Verlag, Berlin
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Smith, R.L., Miller, L.G. & Howes, B.L. The geochemistry of methane in Lake Fryxell, an amictic, permanently ice-covered, antarctic lake. Biogeochemistry 21, 95–115 (1993). https://doi.org/10.1007/BF00000873
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00000873