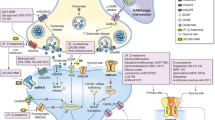
Abstract
The glutamatergic system is the primary excitatory pathway within the CNS and is responsible for cognition, memory, learning, emotion, and mood. Because of its significant importance in widespread nervous system function, it is tightly regulated through multiple mechanisms, such as glutamate recycling, microglial interactions, and inflammatory pathways. Imbalance within the glutamatergic system has been implicated in a wide range of pathological conditions including neurodegenerative conditions, neuromuscular conditions, and mood disorders including depression. Major depressive disorder (MDD) is the most common mood disorder worldwide, has a high prevalence rate, and afflicts approximately 280 million people. While there are numerous treatments for the disease, 30–40% of patients are unresponsive to treatment and deemed treatment resistant; approximately another third experience only partial improvement (World Health Organization, Depression fact sheet [Internet], 2020). Esketamine, the S-enantiomer of ketamine, was approved by the Food and Drug Administration for treatment-resistant depression (TRD) in 2019 and has offered new hope to patients. It is the first treatment targeting the glutamatergic system through a complex mechanism. Numerous studies have implicated imbalance in the glutamatergic system in depression and treatment resistance. Esketamine and ketamine principally work through inhibition of the NMDA receptor, though more recent studies have implicated numerous other mechanisms mediating the antidepressant efficacy of these agents. These mechanisms include increase in brain-derived neurotrophic factor (BDNF), activation of mammalian target of the rapamycin complex (mTORC), and reduction in inflammation. Esketamine and ketamine have been shown to decrease inflammation in numerous ways principally through reducing pro-inflammatory cytokines (e.g., TNF-α, IL-6) (Loix et al., Acta Anaesthesiol Belg 62(1):47–58, 2011; Chen et al., Psychiatry Res 269:207–11, 2018; Kopra et al., J Psychopharmacol 35(8):934–45, 2021). This anti-inflammatory effect has also been shown to be involved in the antidepressive properties of both ketamine and esketamine (Chen et al., Psychiatry Res 269:207–11, 2018; Kopra et al., J Psychopharmacol 35(8):934–45, 2021).
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, et al. Antidepressant effects of ketamine in depressed patients. Biol Psychiatry. 2000;47(4):351–4.
Crupi R, Marino A, Cuzzocrea S. New therapeutic strategy for mood disorders. Curr Med Chem. 2011;18(28):4284–98.
Rosenblat JD, McIntyre RS, Alves GS, Fountoulakis KN, Carvalho AF. Beyond monoamines-novel targets for treatment-resistant depression: a comprehensive review. Curr Neuropharmacol. 2015;13(5):636–55.
World Health Organization. Depression fact sheet [Internet]. 2020. https://www.who.int/news-room/fact-sheets/detail/depression. Accessed 17 Sep 2021.
Center for Disease Control and Prevention. Suicide and self-inflicted injury. 2020. https://www.cdc.gov/nchs/fastats/suicide.htm. Accessed 18 Sep 2021.
Institute of Health Metrics and Evaluation. Health data exchange. 2021. http://ghdx.healthdata.org/gbd-results-tool?params=gbd-api-2019-permalink/d780dffbe8a381b25e1416884959e88b. Accessed 7 Sep 2021.
Bunney WE Jr, Davis JM. Norepinephrine in depressive reactions. A review. Arch Gen Psychiatry. 1965;13(6):483–94.
Delgado PL, Moreno FA. Role of norepinephrine in depression. J Clin Psychiatry. 2000;61(1):5–12.
Hillhouse TM, Porter JH. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp Clin Psychopharmacol. 2015;23(1):1–21.
Cole CE, Patterson RM, Craig JB, Thomas WE, Ristine LP, Stahly M, et al. A controlled study of efficacy of iproniazid in treatment of depression. AMA Arch of Gen Psychiatry. 1959;1(5):513–8.
Fisar Z, Hroudová J, Raboch J. Inhibition of monoamine oxidase activity by antidepressants and mood stabilizers. Neuro Endocrinol Lett. 2010;31(5):645–56.
Gonul AS, Akdeniz F, Taneli F, Donat O, Eker C, Vahip S. Effect of treatment on serum brain-derived neurotrophic factor levels in depressed patients. Eur Arch Psychiatry Clin Neurosci. 2005;255(6):381–6.
Machado-Vieira R, Yuan P, Brutsche N, DiazGranados N, Luckenbaugh D, Manji HK, et al. Brain-derived neurotrophic factor and initial antidepressant response to an N-methyl-D-aspartate antagonist. J Clin Psychiatry. 2009;70(12):1662–6.
Homberg JR, Molteni R, Calabrese F, Riva MA. The serotonin-BDNF duo: developmental implications for the vulnerability to psychopathology. Neurosci Biobehav Rev. 2014;43:35–47.
Björkholm C, Monteggia LM. BDNF—a key transducer of antidepressant effects. Neuropharmacology. 2016;102:72–9.
Buetler L, Clarkin J, Bongar B. Guideline for the systematic treatment of the depressed patient. Oxford Scholarship Online. Oxford: Oxford University Press; 2000.
McIntyre RS, Filteau MJ, Martin L, Patry S, Carvalho A, Cha DS, et al. Treatment-resistant depression: definitions, review of the evidence, and algorithmic approach. J Affect Disord. 2014;156:1–7.
Gaynes B, Asher G, Gartlehner G, Hoffman V, Cokker-Schwimmer E. Definition of treatment-resistant depression in the medicare population. Rockville, MD: Agency for Healthcare Research and Quality (US); 2018.
Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42(1):1–11.
Trullas R, Skolnick P. Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. Eur J Pharmacol. 1990;185(1):1–10.
Curtis DR, Watkins JC. The excitation and depression of spinal neurones by structurally related amino acids. J Neurochem. 1960;6:117–41.
Beneyto M, Kristiansen LV, Oni-Orisan A, McCullumsmith RE, Meador-Woodruff JH. Abnormal glutamate receptor expression in the medial temporal lobe in schizophrenia and mood disorders. Neuropsychopharmacology. 2007;32(9):1888–902.
Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–96.
Zarate C Jr, Machado-Vieira R, Henter I, Ibrahim L, Diazgranados N, Salvadore G. Glutamatergic modulators: the future of treating mood disorders? Harv Rev Psychiatry. 2010;18(5):293–303.
Drago A, Crisafulli C, Sidoti A, Serretti A. The molecular interaction between the glutamatergic, noradrenergic, dopaminergic and serotoninergic systems informs a detailed genetic perspective on depressive phenotypes. Prog Neurobiol. 2011;94(4):418–60.
Andersen JV, Markussen KH, Jakobsen E, Schousboe A, Waagepetersen HS, Rosenberg PA, et al. Glutamate metabolism and recycling at the excitatory synapse in health and neurodegeneration. Neuropharmacology. 2021;196:108719.
Hynd MR, Scott HL, Dodd PR. Glutamate-mediated excitotoxicity and neurodegeneration in Alzheimer’s disease. Neurochem Int. 2004;45(5):583–95.
Kim JS, Schmid-Burgk W, Claus D, Kornhuber HH. Increased serum glutamate in depressed patients. Arch Psychiatr Nervenkr. 1982;232(4):299–304.
Tilleux S, Hermans E. Neuroinflammation and regulation of glial glutamate uptake in neurological disorders. J Neurosci Res. 2007;85(10):2059–70.
Iovino L, Tremblay ME, Civiero L. Glutamate-induced excitotoxicity in Parkinson's disease: the role of glial cells. J Pharmacol Sci. 2020;144(3):151–64.
Li CT, Yang KC, Lin WC. Glutamatergic dysfunction and glutamatergic compounds for major psychiatric disorders: evidence from clinical neuroimaging studies. Front Psych. 2019;9:767.
Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322.
Crane G. Cyloserine as an antidepressant agent. Am J Psychiatry. 1959;115(11):1025–6.
Yüksel C, Öngür D. Magnetic resonance spectroscopy studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68(9):785–94.
Auer DP, Pütz B, Kraft E, Lipinski B, Schill J, Holsboer F. Reduced glutamate in the anterior cingulate cortex in depression: an in vivo proton magnetic resonance spectroscopy study. Biol Psychiatry. 2000;47(4):305–13.
Hasler G, van der Veen JW, Tumonis T, Meyers N, Shen J, Drevets WC. Reduced prefrontal glutamate/glutamine and gamma-aminobutyric acid levels in major depression determined using proton magnetic resonance spectroscopy. Arch Gen Psychiatry. 2007;64(2):193–200.
Block W, Träber F, von Widdern O, Metten M, Schild H, Maier W, et al. Proton MR spectroscopy of the hippocampus at 3 T in patients with unipolar major depressive disorder: correlates and predictors of treatment response. Int J Neuropsychopharmacol. 2009;12(3):415–22.
Clark DL, MacMaster FP, Brown EC, Kiss ZHT, Ramasubbu R. Rostral anterior cingulate glutamate predicts response to subcallosal deep brain stimulation for resistant depression. J Affect Disord. 2020;266:90–4.
Frye MA, Tsai GE, Huggins T, Coyle JT, Post RM. Low cerebrospinal fluid glutamate and glycine in refractory affective disorder. Biol Psychiatry. 2007;61(2):162–6.
Moriguchi S, Takamiya A, Noda Y, Horita N, Wada M, Tsugawa S, et al. Glutamatergic neurometabolite levels in major depressive disorder: a systematic review and meta-analysis of proton magnetic resonance spectroscopy studies. Mol Psychiatry. 2019;24(7):952–64.
Pittenger C, Duman RS. Stress, depression, and neuroplasticity: a convergence of mechanisms. Neuropsychopharmacology. 2008;33(1):88–109.
Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62(1):63–77.
Mateos-Aparicio P, Rodríguez-Moreno A. The impact of studying brain plasticity. Front Cell Neurosci. 2019;13:66.
Barbon A, Popoli M, La Via L, Moraschi S, Vallini I, Tardito D, et al. Regulation of editing and expression of glutamate alpha-amino-propionic-acid (AMPA)/kainate receptors by antidepressant drugs. Biol Psychiatry. 2006;59(8):713–20.
Bleakman D, Alt A, Witkin JM. AMPA receptors in the therapeutic management of depression. CNS Neurol Disord Drug Targets. 2007;6(2):117–26.
Schoepfer R, Monyer H, Sommer B, Wisden W, Sprengel R, Kuner T, et al. Molecular biology of glutamate receptors. Prog Neurobiol. 1994;42(2):353–7.
Du J, Feng L, Yang F, Lu B. Activity- and Ca(2+)-dependent modulation of surface expression of brain-derived neurotrophic factor receptors in hippocampal neurons. J Cell Biol. 2000;150(6):1423–34.
Lee B, Kim Y. The roles of BDNF in the pathophysiology of major depression and in antidepressant treatment. Psychiatry Investig. 2010;7(4):231–5.
Yu H, Chen ZY. The role of BDNF in depression on the basis of its location in the neural circuitry. Acta Pharmacol Sin. 2011;32(1):3–11.
Kowiański P, Lietzau G, Czuba E, Waśkow M, Steliga A, Moryś J. BDNF: a key factor with multipotent impact on brain signaling and synaptic plasticity. Cell Mol Neurobiol. 2018;38(3):579–93.
Dwivedi Y. Brain-derived neurotrophic factor: role in depression and suicide. Neuropsychiatr Dis Treat. 2009;5:433–49.
Murakami S, Imbe H, Morikawa Y, Kubo C, Senba E. Chronic stress, as well as acute stress, reduces BDNF mRNA expression in the rat hippocampus but less robustly. Neurosci Res. 2005;53(2):129–39.
Bliss TV, Cooke SF. Long-term potentiation and long-term depression: a clinical perspective. Clinics (Sao Paulo). 2011;66(1):3–17.
Feyissa AM, Chandran A, Stockmeier CA, Karolewicz B. Reduced levels of NR2A and NR2B subunits of NMDA receptor and PSD-95 in the prefrontal cortex in major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2009;33(1):70–5.
Nowak G, Ordway GA, Paul IA. Alterations in the N-methyl-D-aspartate (NMDA) receptor complex in the frontal cortex of suicide victims. Brain Res. 1995;675(1–2):157–64.
Martinez-Turrillas R, Frechilla D, Del Río J. Chronic antidepressant treatment increases the membrane expression of AMPA receptors in rat hippocampus. Neuropharmacology. 2002;43(8):1230–7.
Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224(1):107–11.
Zhou W, Wang N, Yang C, Li XM, Zhou ZQ, Yang JJ. Ketamine-induced antidepressant effects are associated with AMPA receptors-mediated upregulation of mTOR and BDNF in rat hippocampus and prefrontal cortex. Eur Psychiatry. 2014;29(7):419–23.
Svenningsson P, Tzavara ET, Witkin JM, Fienberg AA, Nomikos GG, Greengard P. Involvement of striatal and extrastriatal DARPP-32 in biochemical and behavioral effects of fluoxetine (Prozac). Proc Natl Acad Sci. 2002;99(5):3182–7.
Liao Y, Tang YL, Hao W. Ketamine and international regulations. Am J Drug Alcohol Abuse. 2017;43(5):495–504.
Corssen G, Domino EF. Dissociative anesthesia: further pharmacologic studies and first clinical experience with the phencyclidine derivative CI-581. Anesth Analg. 1966;45(1):29–40.
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63(8):856–64.
Mathew SJ, Murrough JW, Aan Het Rot M, Collins KA, Reich DL, Charney DS. Riluzole for relapse prevention following intravenous ketamine in treatment-resistant depression: a pilot randomized, placebo-controlled continuation trial. Int J Neuropsychopharmacol. 2010;13(1):71–82.
Ibrahim L, Diazgranados N, Luckenbaugh DA, Machado-Vieira R, Baumann J, Mallinger AG, et al. Rapid decrease in depressive symptoms with an N-methyl-d-aspartate antagonist in ECT-resistant major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2011;35(4):1155–9.
Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Green CE, Perez AM, et al. Antidepressant efficacy of ketamine in treatment-resistant major depression: a two-site randomized controlled trial. Am J Psychiatry. 2013;170(10):1134–42.
Shiroma PR, Johns B, Kuskowski M, Wels J, Thuras P, Albott CS, et al. Augmentation of response and remission to serial intravenous subanesthetic ketamine in treatment resistant depression. J Affect Disord. 2014;155:123–9.
Domino EF. Taming the ketamine tiger—1965. Anesthesiology. 2010;113(3):678–84.
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533(7604):481–6.
Yang C, Ren Q, Qu Y, Zhang JC, Ma M, Dong C, et al. Mechanistic target of rapamycin-independent antidepressant effects of (R)-ketamine in a social defeat stress model. Biol Psychiatry. 2018;83(1):18–28.
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, et al. R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry. 2015;5(9):e632.
Zhang JC, Li SX, Hashimoto K. R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav. 2014;116:137–41.
Daly EJ, Singh JB, Fedgchin M, Cooper K, Lim P, Shelton RC, et al. Efficacy and safety of intranasal esketamine adjunctive to oral antidepressant therapy in treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2018;75(2):139–48.
Daly EJ, Trivedi MH, Janik A, Li H, Zhang Y, Li X, et al. Efficacy of esketamine nasal spray plus oral antidepressant treatment for relapse prevention in patients with treatment-resistant depression: a randomized clinical trial. JAMA Psychiat. 2019;76(9):893–903.
Popova V, Daly EJ, Trivedi M, Cooper K, Lane R, Lim P, et al. Efficacy and safety of flexibly dosed esketamine nasal spray combined with a newly initiated oral antidepressant in treatment-resistant depression: a randomized double-blind active-controlled study. Am J Psychiatry. 2019;176(6):428–38.
Fedgchin M, Trivedi M, Daly EJ, Melkote R, Lane R, Lim P, et al. Efficacy and safety of fixed-dose esketamine nasal spray combined with a new oral antidepressant in treatment-resistant depression: results of a randomized, double-blind, active-controlled study (TRANSFORM-1). Int J Neuropsychopharmacol. 2019;22(10):616–30.
Ochs-Ross R, Daly EJ, Zhang Y, Lane R, Lim P, Morrison RL, et al. Efficacy and safety of esketamine nasal spray plus an oral antidepressant in elderly patients with treatment-resistant depression-TRANSFORM-3. Am J Geriatr Psychiatry. 2020;28(2):121–41.
Spravato Prescribing Information. Spravato (esketamine) [package insert]. Titusville, NJ: Janssen Pharmaceutical; 2019.
Anis NA, Berry SC, Burton NR, Lodge D. The dissociative anaesthetics, ketamine and phencyclidine, selectively reduce excitation of central mammalian neurones by N-methyl-aspartate. Br J Pharmacol. 1983;79(2):565–75.
Martin D, Lodge D. Ketamine acts as a non-competitive N-methyl-D-aspartate antagonist on frog spinal cord in vitro. Neuropharmacology. 1985;24(10):999–1003.
Yamamura T, Harada K, Okamura A, Kemmotsu O. Is the site of action of ketamine anesthesia the N-methyl-D-aspartate receptor? Anesthesiology. 1990;72(4):704–10.
Zanos P, Gould TD. Mechanisms of ketamine action as an antidepressant. Mol Psychiatry. 2018;23(4):801–11.
Andrade C. Ketamine for depression, 4: in what dose, at what rate, by what route, for how long, and at what frequency? J Clin Psychiatry. 2017;78(7):e852–7.
Duman RS, Li N, Liu RJ, Duric V, Aghajanian G. Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 2012;62(1):35–41.
Aleksandrova LR, Phillips AG, Wang YT. Antidepressant effects of ketamine and the roles of AMPA glutamate receptors and other mechanisms beyond NMDA receptor antagonism. J Psychiatry Neurosci. 2017;42(4):222–9.
Orser BA, Pennefather PS, MacDonald JF. Multiple mechanisms of ketamine blockade of N-methyl-D-aspartate receptors. Anesthesiology. 1997;86(4):903–17.
Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, et al. Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature. 2018;554(7692):317–22.
Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. J Neurosci. 2007;27(43):11496–500.
Chowdhury GM, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, et al. Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Mol Psychiatry. 2017;22(1):120–6.
Miller OH, Yang L, Wang CC, Hargroder EA, Zhang Y, Delpire E, et al. GluN2B-containing NMDA receptors regulate depression-like behavior and are critical for the rapid antidepressant actions of ketamine. elife. 2014;3:3581.
Ignácio ZM, Réus GZ, Arent CO, Abelaira HM, Pitcher MR, Quevedo J. New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol. 2016;82(5):1280–90.
Cavalleri L, Merlo Pich E, Millan MJ, Chiamulera C, Kunath T, Spano PF, et al. Ketamine enhances structural plasticity in mouse mesencephalic and human iPSC-derived dopaminergic neurons via AMPAR-driven BDNF and mTOR signaling. Mol Psychiatry. 2018;23(4):812–23.
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–64.
Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology. 2020;45(6):990–7.
Suzuki K, Monteggia LM. The role of eEF2 kinase in the rapid antidepressant actions of ketamine. Adv Pharmacol. 2020;89:79–99.
Zarate CA Jr, Singh JB, Quiroz JA, De Jesus G, Denicoff KK, Luckenbaugh DA, et al. A double-blind, placebo-controlled study of memantine in the treatment of major depression. Am J Psychiatry. 2006;163(1):153–5.
Yamaguchi JI, Toki H, Qu Y, Yang C, Koike H, Hashimoto K, et al. (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology. 2018;43(9):1900–7.
Yang C, Kobayashi S, Nakao K, Dong C, Han M, Qu Y, et al. AMPA receptor activation-independent antidepressant actions of ketamine metabolite (S)-Norketamine. Biol Psychiatry. 2018;84(8):591–600.
Morris PJ, Moaddel R, Zanos P, Moore CE, Gould TD, Zarate CA Jr, et al. Synthesis and N-methyl-d-aspartate (NMDA) receptor activity of ketamine metabolites. Org Lett. 2017;19(17):4572–5.
Pfenninger EG, Durieux ME, Himmelseher S. Cognitive impairment after small-dose ketamine isomers in comparison to equianalgesic racemic ketamine in human volunteers. Anesthesiology. 2002;96(2):357–66.
Bahji A, Vazquez GH, Zarate CA Jr. Comparative efficacy of racemic ketamine and esketamine for depression: a systematic review and meta-analysis. J Affect Disord. 2021;278:542–55.
Fukumoto K, Toki H, Iijima M, Hashihayata T, Yamaguchi JI, Hashimoto K, et al. Antidepressant potential of (R)-ketamine in rodent models: comparison with (S)-ketamine. J Pharmacol Exp Ther. 2017;361(1):9–16.
Fu DJ, Ionescu DF, Li X, Lane R, Lim P, Sanacora G, et al. Esketamine nasal spray for rapid reduction of major depressive disorder symptoms in patients who have active suicidal ideation with intent: double-blind, randomized study (ASPIRE I). J Clin Psychiatry. 2020;81(3):19m13191. https://doi.org/10.4088/JCP.19m13191.
Ionescu DF, Fu DJ, Qiu X, Lane R, Lim P, Kasper S, et al. Esketamine nasal spray for rapid reduction of depressive symptoms in patients with major depressive disorder who have active suicide ideation with intent: results of a phase 3, double-blind, randomized study (ASPIRE II). Int J Neuropsychopharmacol. 2021;24(1):22–31.
Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biol Psychiatry. 2009;65(9):732–41.
Miller AH, Raison CL. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol. 2016;16(1):22–34.
Ader R, Cohen N. Behaviorally conditioned immunosuppression. Psychosom Med. 1975;37(4):333–40.
Plaut M. Lymphocyte hormone receptors. Annu Rev Immunol. 1987;5:621–69.
Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2(4):269–76.
Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, et al. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15(3):199–226.
Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, et al. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci. 2003;100(4):1920–5.
Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163(9):1630–3.
Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27(1):24–31.
Brydon L, Harrison NA, Walker C, Steptoe A, Critchley HD. Peripheral inflammation is associated with altered substantia nigra activity and psychomotor slowing in humans. Biol Psychiatry. 2008;63(11):1022–9.
Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67(5):446–57.
Kopra E, Mondelli V, Pariante C, Nikkheslat N. Ketamine’s effect on inflammation and kynurenine pathway in depression: a systematic review. J Psychopharmacol. 2021;35(8):934–45.
Kim H, Chen L, Lim G, Sung B, Wang S, McCabe MF, et al. Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J Clin Invest. 2012;122(8):2940–54.
Dobos N, de Vries EF, Kema IP, Patas K, Prins M, Nijholt IM, et al. The role of indoleamine 2,3-dioxygenase in a mouse model of neuroinflammation-induced depression. J Alzheimers Dis. 2012;28(4):905–15.
Müller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12(11):988–1000.
Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91(1):220–9.
Myint AM, Schwarz MJ, Müller N. The role of the kynurenine metabolism in major depression. J Neural Transm. 2012;119(2):245–51.
Stone TW, Perkins MN. Quinolinic acid: a potent endogenous excitant at amino acid receptors in CNS. Eur J Pharmacol. 1981;72(4):411–2.
Gibney SM, McGuinness B, Prendergast C, Harkin A, Connor TJ. Poly I:C-induced activation of the immune response is accompanied by depression and anxiety-like behaviours, kynurenine pathway activation and reduced BDNF expression. Brain Behav Immun. 2013;28:170–81.
Frommberger UH, Bauer J, Haselbauer P, Fräulin A, Riemann D, Berger M. Interleukin-6-(IL-6) plasma levels in depression and schizophrenia: comparison between the acute state and after remission. Eur Arch Psychiatry Clin Neurosci. 1997;247(4):228–33.
Yirmiya R, Pollak Y, Morag M, Reichenberg A, Barak O, Avitsur R, et al. Illness, cytokines, and depression. Ann N Y Acad Sci. 2000;917:478–87.
Rethorst CD, Toups MS, Greer TL, Nakonezny PA, Carmody TJ, Grannemann BD, et al. Pro-inflammatory cytokines as predictors of antidepressant effects of exercise in major depressive disorder. Mol Psychiatry. 2013;18(10):1119–24.
Jia Y, Liu L, Sheng C, Cheng Z, Cui L, Li M, et al. Increased serum levels of cortisol and inflammatory cytokines in people with depression. J Nerv Ment Dis. 2019;207(4):271–6.
Müller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Müller B, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11(7):680–4.
Akhondzadeh S, Jafari S, Raisi F, Nasehi AA, Ghoreishi A, Salehi B, et al. Clinical trial of adjunctive celecoxib treatment in patients with major depression: a double blind and placebo controlled trial. Depress Anxiety. 2009;26(7):607–11.
Krause D, Myint AM, Schuett C, Musil R, Dehning S, Cerovecki A, et al. High Kynurenine (a tryptophan metabolite) predicts remission in patients with major depression to add-on treatment with Celecoxib. Front Psych. 2017;8:16.
Halaris A, Cantos A, Johnson K, Hakimi M, Sinacore J. Modulation of the inflammatory response benefits treatment-resistant bipolar depression: a randomized clinical trial. J Affect Disord. 2020;261:145–52.
Malaguarnera M, Di Fazio I, Restuccia S, Pistone G, Ferlito L, Rampello L. Interferon alpha-induced depression in chronic hepatitis C patients: comparison between different types of interferon alpha. Neuropsychobiology. 1998;37(2):93–7.
Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344(13):961–6.
Raison CL, Borisov AS, Majer M, Drake DF, Pagnoni G, Woolwine BJ, et al. Activation of central nervous system inflammatory pathways by interferon-alpha: relationship to monoamines and depression. Biol Psychiatry. 2009;65(4):296–303.
Schiepers OJ, Wichers MC, Maes M. Cytokines and major depression. Prog Neuro-Psychopharmacol Biol Psychiatry. 2005;29(2):201–17.
Owens MJ, Nemeroff CB. Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev. 1991;43(4):425–73.
Pariante CM, Miller AH. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49(5):391–404.
Claes SJ. CRH, stress, and major depression: a psychobiological interplay. Vitam Horm. 2004;69:117–50.
Holsboer F, Ising M. Central CRH system in depression and anxiety—evidence from clinical studies with CRH1 receptor antagonists. Eur J Pharmacol. 2008;583(2–3):350–7.
Musazzi L, Racagni G, Popoli M. Stress, glucocorticoids and glutamate release: effects of antidepressant drugs. Neurochem Int. 2011;59(2):138–49.
van Tol MJ, van der Wee NJ, van den Heuvel OA, Nielen MM, Demenescu LR, Aleman A, et al. Regional brain volume in depression and anxiety disorders. Arch Gen Psychiatry. 2010;67(10):1002–11.
Loix S, De Kock M, Henin P. The anti-inflammatory effects of ketamine: state of the art. Acta Anaesthesiol Belg. 2011;62(1):47–58.
Van der Linden P, Gilbart E, Engelman E, Schmartz D, de Rood M, Vincent JL. Comparison of halothane, isoflurane, alfentanil, and ketamine in experimental septic shock. Anesth Analg. 1990;70(6):608–17.
Yli-Hankala A, Kirvelä M, Randell T, Lindgren L. Ketamine anaesthesia in a patient with septic shock. Acta Anaesthesiol Scand. 1992;36(5):483–5.
Lange M, Bröking K, van Aken H, Hucklenbruch C, Bone HG, Westphal M. Role of ketamine in sepsis and systemic inflammatory response syndrome. Anaesthesist. 2006;55(8):883–91.
Chen MH, Li CT, Lin WC, Hong CJ, Tu PC, Bai YM, et al. Rapid inflammation modulation and antidepressant efficacy of a low-dose ketamine infusion in treatment-resistant depression: a randomized, double-blind control study. Psychiatry Res. 2018;269:207–11.
Kiraly DD, Horn SR, Van Dam NT, Costi S, Schwartz J, Kim-Schulze S, et al. Altered peripheral immune profiles in treatment-resistant depression: response to ketamine and prediction of treatment outcome. Transl Psychiatry. 2017;7(3):1065.
Dale O, Somoyogi AA, Yiba L, Sullivan T, Shavit Y. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg. 2012;115(4):934–43.
Haroon E, Daguanno AW, Woolwine BJ, Goldsmith DR, Baer WM, Wommack EC, et al. Antidepressant treatment resistance is associated with increased inflammatory markers in patients with major depressive disorder. Psychoneuroendocrinology. 2018;95:43–9.
Zhang GF, Wang J, Han JF, Guo J, Xie ZM, Pan W, et al. Acute single dose of ketamine relieves mechanical allodynia and consequent depression-like behaviors in a rat model. Neurosci Lett. 2016;631:7–12.
Verdonk F, Petit AC, Abdel-Ahad P, Vinckier F, Jouvion G, de Maricourt P, et al. Microglial production of quinolinic acid as a target and a biomarker of the antidepressant effect of ketamine. Brain Behav Immun. 2019;81:361–73.
Wang W, Liu L, Yang X, Gao H, Tang QK, Yin LY, et al. Ketamine improved depressive-like behaviors via hippocampal glucocorticoid receptor in chronic stress induced- susceptible mice. Behav Brain Res. 2019;364:75–84.
Johnston CJ, Fitzgerald PJ, Gewarges JS, Watson BO, Spencer-Segal JL. Ketamine decreases HPA axis reactivity to a novel stressor in male but not female mice. bioRxiv. 2021.
Walker AJ, Foley BM, Sutor SL, McGillivray JA, Frye MA, Tye SJ. Peripheral proinflammatory markers associated with ketamine response in a preclinical model of antidepressant-resistance. Behav Brain Res. 2015;293:198–202.
Rong C, Park C, Rosenblat JD, Subramaniapillai M, Zuckerman H, Fus D, et al. Predictors of response to ketamine in treatment resistant major depressive disorder and bipolar disorder. Int J Environ Res Public Health. 2018;15(4):771.
National Institute of Health. Clinicaltrials.gov: treatment resistant depression [Internet]. 2021. Clinicaltrials.gov.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Singapore Pte Ltd.
About this chapter
Cite this chapter
Halaris, A., Cook, J. (2023). The Glutamatergic System in Treatment-Resistant Depression and Comparative Effectiveness of Ketamine and Esketamine: Role of Inflammation?. In: Kim, YK. (eds) Neuroinflammation, Gut-Brain Axis and Immunity in Neuropsychiatric Disorders. Advances in Experimental Medicine and Biology, vol 1411. Springer, Singapore. https://doi.org/10.1007/978-981-19-7376-5_21
Download citation
DOI: https://doi.org/10.1007/978-981-19-7376-5_21
Published:
Publisher Name: Springer, Singapore
Print ISBN: 978-981-19-7375-8
Online ISBN: 978-981-19-7376-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)