Abstract
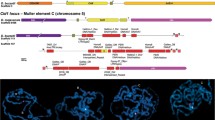
Sdic is a new gene that evolved recently in the lineage of Drosophila melanogaster. It was formed from a duplication and fusion of the gene AnnX, which encodes annexin X, and Cdic, which encodes the intermediate polypeptide chain of the cytoplasmic dynein. The fusion joins AnnX exon 4 with Cdic intron 3, which brings together three putative promoter elements for testes- specific expression of Sdic: the distal conserved element (DCE) and testesspecific element (TSE) are derived from AnnX, and the proximal conserved element (PCE) from Cdic intron 3. Sdic transcription initiates within the PCE, and translation is initiated within the sequence derived from Cdic intron 3, continuing through a 10 base pair insertion that creates a new splice donor site that enables the new coding sequence derived from intron 3 to be joined with the coding sequence of Cdic exon 4. A novel protein is created lacking 100 residues at the amino end that contain sequence motifs essential for the function of cytoplasmic dynein intermediate chains. Instead, the amino end is a hydrophobic region of 16 residues that resembles the amino end of axonemal dynein intermediate chains from other organisms. The downstream portion of Sdic features large deletions eliminating Cdic exons v2 and v3, as well as multiple frameshift deletions or insertions. The new protein becomes incorporated into the tail of the mature sperm and may function as an axonemal dynein intermediate chain. The new Sdic gene is present in about 10 tandem repeats between the wildtype Cdic and AnnX genes located near the base of the X chromosome. The implications of these findings are discussed relative to the origin of new gene functions and the process of speciation.
The authors Jose María Ranz and Ana Rita Ponce contributed equally to this work.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
Abbreviations
- dynein IC:
-
dynein intermediate polypeptide chain
- DCE:
-
distal conserved element
- PCE:
-
proximal conserved element
- TSE:
-
testes-specific element
References
Aniento, F., N. Emans, G. Griffiths & J. Gruenberg, 1993. Cytoplasmic dynein-dependent vesicular transport from early to late endosomes. J. Cell Biol. 123: 1373–1387.
Aravin, A.A., N.M. Naumova, A.V. Tulin, V.V. Vagin, Y.M. Rozovsky & V.A. Gvozdev, 2001. Double-stranded RNA-mediated silencing of genomic tandem repeats and transpos-able elements in the D. melanogaster germline. Curr. Biol. 11: 1017–1027.
Atlan, A., H. Mercot, C. Landre & C. Montchampmoreau, 1997. The sex-ratio trait in Drosophila simulans: geographical distribution of distortion and resistance. Evolution 51: 1886–1895.
Balakireva, M.D., Y.Y. Shevelyov, D.I. Nurminsky, K.J. Livak & V.A. Gvozdev, 1992. Structural organization and diversification of Y-linked sequences comprising Su(Ste) genes in Drosophila melanogaster. Nucl. Acids Res. 20: 3731–3736.
Barton, G.J., R.H. Newman, P.S. Freemont & M.J. Crumpton, 1991. Amino acid sequence analysis of the annexin super-gene family of proteins. Eur. J. Biochem. 198: 749–760.
Begun, D.J., 1997. Origin and evolution of a new gene descended from alcohol dehydrogenase in Drosophila. Genetics 145: 375–382.
Bozzetti, M.R., S. Massari, P. Finelli, F. Meggio, L.A. Pinna, B. Boldyreff, O.G. Issinger, G. Palumbo, C. Ciriaco, S. Bonaccorsi & S. Pimpinelli, 1995. The Ste locus, a component of the parasitic cry-ste system of Drosophila melanogaster, encodes a protein that forms crystals in primary spermatocytes and mimics properties of the beta subunit of casein kinase. Proc. Natl. Acad. Sci. USA 92: 6067–6071.
Civetta, A. & R.S. Singh, 1995. High divergence of reproductive tract proteins and their association with postzygotic reproductive isolation in Drosophila melanogaster and Drosophila virilis group species. J. Mol. Evol. 41: 1085–1095.
Corthesy-Theulaz, I., A. Pauloin & S.R. Rfeffer, 1992. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J. Cell. Biol. 118: 1333–1345.
Coulthart, M.B. & R.S. Singh, 1988. High level of divergence of male-reproductive-tract proteins between Drosophila melanogaster and its sibling species, D. simulans. Mol. Biol. Evol. 5: 182–191.
Dillman, J.F., L.P. Dabney & K.K. Pfister, 1996. Cytoplasmic dynein is associated with slow axonal transport. Proc. Natl. Acad. Sci. USA 93: 141–144.
Geisow, M.J., 1991. Annexins: forms without function but not without fun. Trends Biotechnol. 9: 180–181.
Gilbert, W., 1978. Why genes in pieces? Nature 271: 501.
Jeffs, P.S., E.C. Holmes & M. Ashburner, 1994. The molecular evolution of the alcohol dehydrogenase and alcohol dehydrogenase-related genes in the Drosophila melanogaster species subgroup. Mol. Biol. Evol. 11: 287–304.
King, S.M., E. Barbarese, J.F. Dillman, R.S. Patel-King, J.H. Carson & K.K. Pfister, 1996. Brain cytoplasmic and flagellar outer arm dyneins share a highly conserved Mr 8,000 light chain. J. Biol. Chem. 271: 19358–19366.
Laurie, C.C., 1997. The weaker sex is heterogamatic: 75 years of Haldane’s rule. Genetics 147: 937–951.
Livak, K.J., 1990. Detailed structure of the Drosophila melanogaster Stellate genes and their transcripts. Genetics 124: 303–316.
Long, M., 2001. Evolution of novel genes. Curr. Opin. Genet. Dev. 11:673–680.
Long, M. & C.H. Langley, 1993. Natural selection and the origin of jingwei, a chimeric processed functional gene in Drosophila. Science 260: 91–95.
Long, M., C. Rosenberg & W. Gilbert, 1995. Intron phase correlations and the evolution of the intron/exon structure of genes. Proc. Natl. Acad. Sci. USA 92.
Luque, T., G. Marfany & R. Gonzàlez-Duarte, 1997. Characterization and molecular analysis of Adh retrosequences in species of the Drosophila obscura group. Mol. Biol. Evol. 14: 1316–1325.
Ma, S., L. Trivinos-Lagos, R. Graf & R.L. Chisholm, 1999. Dynein intermediate chain mediated dynein-dynactin interaction is required for interphase microtubule organization and centrosome replication and separation in Dictyostelium. J. Cell Biol. 147: 1261–1273.
Martinez-Cruzado, J.C., C. Swimmer, M.G. Fenerjian & F.C. Kafatos, 1988. Evolution of the autosomal chorion locus in Drosophila. I. General organization of the locus and sequence comparisons of genes s15 and s19 in evolutionary distant species. Genetics 199: 663–677.
Mazumdar, M., A. Mikami, M.A. Gee & R.B. Vallee, 1996. In vitro motility from recombinant dynein heavy chain. Proc. Natl. Acad. Sci. USA 93: 6552–6556.
McClean, J.R., C.J. Merrill, P.A. Powers & B. Ganetzky, 1994. Functional identification of the segregation distorter locus of Drosophila melanogaster by germline transformation. Genetics 137:201–209.
Mckee, B.D. & M.T. Satter, 1996. Structure of the Y chromosomal Su(Ste) locus in Drosophila melanogaster and evidence for localized recombination among repeats. Genetics 142: 149–161.
Michiels, F., A. Gasch, B. Kaltschmidt & R. Renkawitz-Pohl, 1989. A 14bp promoter element directs the testis specificity of the Drosophila beta 2 tubulin gene. EMBO J. 8: 1559–1565.
Nurminsky, D.I., E.N. Moriyama, E.R. Lozovskaya & D.L. Haiti, 1995. Molecular phylogeny and genome evolution in the Drosophila virilis group: duplications of the alcohol dehydrogenase gene. Mol. Biol. Evol. 13: 132–149.
Nurminsky, D.I., E.V. Benevolenskaya, M.V. Nurminskaya, Y.Y. Shevelyov, D.L. Haiti & V.A. Gvozdev, 1998a. Cytoplasmic dynein intermediate chain isoforms with different targeting properties created by tissue-specific alternative splicing. Mol. Cell. Biol. 18:6816–6825.
Nurminsky, D.I., M.V. Nurminskaya, D. De Aguiar & D.L. Hartl, 1998b. Selective sweep of a newly evolved sperm-specific gene in Drosophila. Nature 396: 572–575.
Nurminsky, D., D. De Aguiar, C.D. Bustamante & D.L. Hartl, 2001. Chromosomal effects of rapid gene evolution in Drosophila melanogaster. Science 291: 128–130.
Palumbo, G., S. Bonaccorsi, L.G. Robbins & S. Pimpinelli, 1994. Genetic analysis of stellate elements of Drosophila melanogaster. Genetics 138: 1181–1197.
Paschal, B.M., A. Mikami, K.K. Pfister & R.B. Vallee, 1992. Homology of the 74-kD cytoplasmic dynein subunit with a flagellar dynein polypeptide suggests an intracellular targeting function. J. Cell Biol. 118: 1133–1143.
Petrov, D.A. & D.L. Haiti, 1997. Trash DNA is what gets thrown away: high rate of DNA loss in Drosophila. Gene 205: 279–289.
Petrov, D.A. & D.L. Haiti, 1998. High rate of DNA loss in the D. melanogaster and D. virilis species groups. Mol. Biol. Evol. 15: 293–302.
Petrov, D.A., E.R. Lozovskaya & D.L. Hartl, 1996. High intrinsic rate of DNA loss in Drosophila. Nature 384: 346–349.
Robin, C., R.J. Russell, K.M. Medveczky & J.G. Oakeshott, 1996. Duplication and divergence of the genes of the esterase cluster of D. melanogaster. J. Mol. Evol. 43: 241–252.
Russell, S.R.H. & K. Kaiser, 1994. A Drosophila melanogaster chromosome-2L repeat is expressed in the male germ line. Chromosoma 103: 63–72.
Schroer, T.A., E.R. Steuer & M.P. Sheetz, 1989. Cytoplasmic dynein is a minus end-directed motor for membranous organelles. Cell 7:331–343.
Snyder, M. & N. Davidson, 1983. Two gene families clustered in a small region of the Drosophila genome. J. Mol. Biol. 166: 101–118.
Steffen, W., S. Karki, K.T. Vaughan, R.B. Vallee, E.L.F. Holzbaur, D.G. Weiss & S.A. Kuznetsov, 1997. The involvement of the intermediate chain of cytoplasmic dynein in binding the motor complex to membranous organelles of Xenopus oocytes. Mol. Biol. Cell 8: 2077–2088.
Steinemann, M. & S. Steinemann, 1990. Evolutionary changes in the organization of the major Lcp gene cluster during sex chromosomal differentiation in the sibling species Drosophila persimilis, D. pseudoobscura and D. miranda. Chromosoma 99: 424–431.
Thomas, S. & R.S. Singh, 1992. A comprehensive study of genetic variation in natural population of Drosophila melanogaster. VII. Varying rates of genic divergence as revealed by two-dimensional electrophoresis. Mol. Biol. Evol. 9: 507–525.
Ting, C.T., S.C. Tsaur & C.I. Wu, 2000. The phylogeny of closely related species as revealed by the genealogy of a speciation gene, Odysseus. Proc. Natl. Acad. Sci. USA 97: 5313–5316.
Ting, C.T., S.C. Tsaur, M.L. Wu & C.I. Wu, 1998. A rapidly evolving homeobox at the site of a hybrid sterility gene. Science 282: 1501–1504.
Vaisberg, E.A., M.P. Koonce & J.R. McIntosh, 1993. Cytoplasmic dynein plays a role in mammalian mitotic spindle formation. J. Cell Biol. 123:849–858.
Vieira, C.P., J. Vieira & D.L. Hartl, 1997. The evolution of small gene clusters: evidence for an independent origin of the maltase gene cluster in D. virilis and D. melanogaster. Mol. Biol. Evol. 14: 985–993.
Wang, W., J.M. Zhang, C Alvarez, A. Llopart & M. Long, 2000. The origin of the Jingwei gene and the complex modular structure of its parental gene, yellow emperor, in Drosophila melanogaster. Mol. Biol. Evol. 17: 1294–1301.
Wilkerson, C.G., S.M. King, A. Koutoulis, G.J. Pazour & G.B. Witman, 1995. The 78,000 M(r) intermediate chain of Chlamydomonas outer arm dynein is a WD-repeat protein required for arm assembly. J. Cell Biol. 129: 169–178.
Wu, C.-I. & A.W. Davis, 1993. Evolution of postmating reproductive isolation: the composite nature of Haldane’s rule and its genetic bases. Am. Nat. 142: 187–212.
Wu, C.-I., N.A. Johnson & M.F. Palopoli, 1996. Haldane’s rule and its legacy: why are there so many sterile males? Trends Ecol. Evol. 11:281–284.
Wu, C.-I., T.W. Lyttle, M.-L. Wu & G.-F. Lin, 1988. Association between a satellite DNA sequence and the Responder of Segregation Distorter in D. melanogaster. Cell 54: 179–189.
Xiang, X., S.M. Beckwith & N.R. Morris, 1994. Cytoplasmic dynein is involved in nuclear migration in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 91: 2100–2104.
Author information
Authors and Affiliations
Editor information
Rights and permissions
Copyright information
© 2003 Springer Science+Business Media Dordrecht
About this chapter
Cite this chapter
Ranz, J.M., Ponce, A.R., Hartl, D.L., Nurminsky, D. (2003). Origin and evolution of a new gene expressed in the Drosophila sperm axoneme. In: Long, M. (eds) Origin and Evolution of New Gene Functions. Contemporary Issues in Genetics and Evolution, vol 10. Springer, Dordrecht. https://doi.org/10.1007/978-94-010-0229-5_12
Download citation
DOI: https://doi.org/10.1007/978-94-010-0229-5_12
Publisher Name: Springer, Dordrecht
Print ISBN: 978-94-010-3982-6
Online ISBN: 978-94-010-0229-5
eBook Packages: Springer Book Archive