Abstract
Autophagy usually functions in cell-protective events. However, it may also be utilized as a cell-suicide mechanism, which is known as “autophagic cell death.” Autophagic cell death is frequently induced in cells that lack apoptotic machinery, such as p53-deficient cancer cells. Therefore, small compounds that activate autophagic cell death are good candidates for anticancer chemotherapeutics to combat p53-deficient cancers. This chapter focuses on recent advances in autophagy/autophagic cell death and their relationship with tumorigenesis.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
Apoptosis and Other Types of Cell Death
Apoptosis is a form of programmed cell death (PCD) and its molecular basis is well understood. Mammalian cells possess two apoptotic signaling pathways, which are known as the intrinsic and extrinsic pathways. In the intrinsic pathway, mitochondria play a crucial role by increasing membrane permeability to release several apoptogenic molecules (e.g., cytochrome c, Smac, and Omi) into the cytoplasm. After its release, cytochrome c associates with Apaf-1, which activates the caspase cascade to execute apoptotic cell death. Smac and Omi accelerate caspase activation by inhibiting IAP family proteins that function as endogenous caspase inhibitors. The Bcl-2 family proteins are well-characterized regulators of apoptosis, which directly modulate mitochondrial membrane permeability [1]. This family of proteins contains anti-apoptotic members, such as Bcl-2 and Bcl-xL, and pro-apoptotic members, including multidomain Bax and Bak, as well as numerous BH3-only proteins. Multidomain pro-apoptotic Bax and Bak are functionally redundant and play a direct role in increasing mitochondrial membrane permeability, thereby leading to the release of apoptogenic proteins. BH3-only proteins function as death transducers by activating Bax/Bak or inactivating anti-apoptotic Bcl-2 family members [1].
Until 10 years ago, the evidence indicated that apoptosis is the primary mediator of physiological and pathological cell death. However, a detailed analysis of Bax/Bak double-knockout mice with a totally inhibited intrinsic apoptotic pathway showed that PCD mainly proceeds normally in organisms that lack apoptosis [2, 3]. These findings encouraged researchers to identify other pathways of PCD, i.e., non-apoptotic cell death. Several types of non-apoptotic cell death have been elucidated, such as programmed necrosis [4] and autophagic cell death [5]. Previously, necrosis was considered to be a form of accidental cell death due to physicochemical stressors but recent evidence has demonstrated the existence of regulated (or programmed) necrosis. Regulated necrosis is a genetically controlled form of cell death, which is characterized morphologically by cytoplasmic granulation and cellular swelling. Necroptosis is one of the best-characterized types of programmed necrosis, which is activated by several kinases, including RIP1, RIP3, and MLKL, whereas it is inhibited by the small compound necrostatin-1 [6]. Autophagic cell death is another type of non-apoptotic cell death that occurs via autophagy activation. Autophagy has long been considered a cell-protective mechanism but recent evidence indicates that the hyperactivation of autophagy is sometimes utilized as a cell-suicide mechanism. This chapter summarizes the current knowledge of autophagy and autophagic cell death.
Autophagy
Autophagy is a catabolic process that digests cellular contents within lysosomes. Autophagy is a low-level constitutive function, which is accelerated by a variety of cellular stressors such as nutrient starvation, DNA damage, and organelle damage. Autophagy is a protective mechanism that facilitates the degradation of superfluous or damaged cellular constituents, although hyperactivation of autophagy can lead to cell death.
In this multistep process, autophagy substrates, such as cytoplasm and damaged organelles, are sequestered inside isolation membranes, which eventually mature into double-membrane structures called autophagosomes. Autophagosomes subsequently fuse with lysosomes to form autolysosomes where the sequestered components are digested (Fig. 1) [7]. The molecular basis of autophagy was elucidated in autophagy-defective mutant yeasts [8]. The subsequent identification of vertebrate homologs greatly expanded our understanding of the molecular mechanisms of autophagy.
Hypothetical model of macroautophagy. There are at least two modes of macroautophagy, i.e., conventional and alternative macroautophagy. Conventional macroautophagy depends on Atg5 and Atg7, is associated with LC3 modification, and may originate from ER-mitochondria contact membrane. In contrast, alternative macroautophagy occurs independent of Atg5 or Atg7 expression and LC3 modification. The generation of autophagic vacuoles in this type of macroautophagy may originate from Golgi membranes and late endosomes (LE) in a Rab9-dependent manner. Although both of these processes lead to bulk degradation of damaged proteins or organelles by generating autolysosomes, they seem to be activated by different stimuli in different cell types and have different physiological roles
Autophagy is driven by over 30 proteins (Atgs), which are well conserved from yeasts to mammals [7]. Atg1 [also called Unc51-like kinase 1 (Ulk1)] is a serine/threonine kinase that is essential for the initiation of autophagy [9]. Autophagy is also regulated by phosphatidylinositol 3-kinase (PI3K) type III, which is a component of a multi-protein complex that includes Atg6 (Beclin1). PI3K promotes invagination of the membrane at domains rich in phosphatidylinositol-3-phosphate, which are called omegasomes, thereby initiating the generation of the isolation membrane [10]. The subsequent expansion and closure of isolation membranes are mediated by two ubiquitin-like conjugation pathways: the Atg5–Atg12 pathway and the MAP–LC3 pathway [7]. In cells that lack these ubiquitin-like conjugation systems, such as Atg5- and Atg7-deficient cells, autophagosome formation is largely disrupted, which indicates the necessity for Atg5 and Atg7 in autophagy. Ubiquitin-like conjugation of phosphatidylethanolamine to LC3 facilitates the translocation of LC3 from the cytosol to the sites of origin of the autophagic membrane. This form of translocation is recognized as a reliable marker of autophagy.
Alternative Macroautophagy
Although Atg5 has long been considered as an essential molecule for autophagy, we recently identified an Atg5-independent type of autophagy, which is induced when cells are severely stressed, such as in the event of DNA damage. The morphology of Atg5-independent autophagic structures is indistinguishable from that of those formed during Atg5-dependent autophagy, i.e., isolation membranes, autophagosomes, and autolysosomes [11]. Thus, we designated this form of Atg5-independent autophagy as “alternative macroautophagy” (Fig. 1). This alternative macroautophagy is driven by Ulk1 and PI3K complexes during the initiation steps. These molecules also function during the initiation of conventional autophagy. However, this pathway is not mediated by other components such as Atg9 and proteins in the ubiquitin-like protein conjugation system (Atg5, Atg7, and LC3), which extend conventional autophagic membranes. Alternative macroautophagy requires the extension of autophagic membranes, thus several unidentified molecules may mediate this function.
Alternative macroautophagy is not an atypical form of autophagy because it occurs in a wide variety of cells, including embryonic fibroblasts and thymocytes, as well as in several tissues such as the heart, brain, and liver [11]. Erythrocyte maturation is a representative example of a physiological alternative macroautophagy. Erythrocytes undergo enucleation and clearance of mitochondria during terminal differentiation, and the possible involvement of autophagy in the latter process was proposed on the basis of a morphological analysis. However, the maturation process is normal in Atg5-deficient erythrocytes. In agreement, an ultrastructural analysis showed that mitochondria are also engulfed and digested by autophagic structures in both the wild-type and Atg5-deficient reticulocytes. These results indicate that conventional macroautophagy does not eliminate mitochondria from erythrocytes. In contrast, mitochondrial clearance is significantly reduced in Ulk1-deficient reticulocytes where alternative macroautophagy is absent. These data suggest that Ulk1-mediated alternative macroautophagy plays a pivotal role in the elimination of mitochondria from erythrocytes [12, 13]. Ongoing research should provide a more detailed picture of the physiological and pathological relevance of alternative macroautophagy in the near future.
Autophagic Cell Death
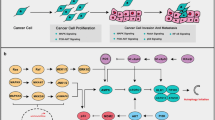
Autophagy is activated by most cellular stressors, thus numerous autophagy-containing cells are often observed in regions where cell death occurs (Fig. 2). In most cases, autophagy has a cell protection function against cellular stressors, but autophagy is utilized as a cell-suicide mechanism in some cases [5]. Certain modes of cell death that are accompanied by protective autophagy are not referred to as “autophagic cell death.” In particular, the term “autophagic cell death” should be used when cell death is performed via autophagy activation. This can be demonstrated by the suppression of cell death by autophagy inhibitors (e.g., 3-methyl adenine and wortmannin) or by the genetic ablation of autophagy (e.g., knockout or siRNA silencing of essential autophagy genes). If autophagy inhibition does not prevent cell death, this process should not be referred to as autophagic cell death.
Molecular mechanism of apoptosis and autophagic cell death. An increase in the permeability of the outer mitochondrial membrane is crucial for apoptosis to occur and is regulated by multidomain pro-apoptotic members of the Bcl-2 family (Bax and Bak), resulting in the release of cytochrome c into the cytoplasm. Then, cytochrome c associates with Apaf-1, which activates the caspase cascade to execute apoptotic cell death. Apoptosis-associated mitochondrial membrane permeability is primarily controlled by Bcl-2 family members. When apoptosis is blocked, various apoptotic stimuli activate autophagy, resulting in the induction of autophagic cell death
Autophagic cell death occurs in several distinct settings. First, caspase-independent, autophagy-dependent PCD occurs during mammalian embryogenesis. Second, autophagy may induce developmental cell death during regression of the salivary glands in Drosophila [14]. Third, autophagy-mediated cell death is induced in apoptosis-resistant cells, particularly in the absence of the pro-apoptotic proteins Bax and Bak [5]. The latter situation should provide an excellent experimental system for investigating autophagic cell death because the process can be observed at the cellular level.
Bax/Bak-deficient cells do not undergo apoptosis after exposure to a variety of apoptotic stimuli, although these cells still die in numerous autophagic structures (Fig. 2). This type of cell death is inhibited by autophagy inhibitors or by silencing autophagy genes such as Atg5 and Atg6. Thus, Atg5-dependent autophagy is required for the death of Bax/Bak-deficient cells after exposure to apoptotic stimuli [5]. Although we discovered Atg5-independent alternative autophagy, it is not involved in the autophagic cell death of Bax/Bak-deficient cells. However, this does not necessarily indicate that alternative autophagy is irrelevant for autophagic cell death. In other situations, alternative autophagy may potentially induce autophagic cell death.
Cancer and Autophagic Cell Death
It has been suggested that autophagic cell death may participate in physiological and pathological events. A large body of evidence indicates that inhibition of apoptosis is critical for tumorigenesis, but the elimination of cancer cells may also be mediated by autophagic cell death, and there is evidence that decreased autophagic activity is related to tumorigenesis. For example, the levels of Beclin-1 (Atg6) are lower in some types of cancers of the ovary, breast, and prostate because of monoallelic mutations [15]. Furthermore, mice that are heterozygous for the beclin-1 gene are cancer prone [15, 16], which strongly suggests that inhibition of Beclin-1 expression contributes to the pathogenesis of cancer. Moreover, Atg5, LC3, and Fip200 are associated with myeloma, glioblastoma, and breast cancer, respectively [17, 18], thereby suggesting that the failure of cells to undergo autophagy leads to tumor progression.
Several mechanisms may explain how tumorigenesis is mediated by the failure of cells to undergo autophagy: (1) accumulation of p62, a substrate of autophagy, leads to NF-κB activation [19]; (2) accumulation of p62 stabilizes Nrf2, which makes tumor cells resistant to hypoxic stress [20]; (3) retention of damaged organelles, including mitochondria, increases the level of active oxygen species and increases the mutation rate; and (4) defective elimination of cancer cells due to the loss of autophagic cell death [21]. These mechanisms may be cell- and stimulus-type specific; however, we believe that it is reasonable to conclude that the failure of autophagic cell death is one of the most crucial mechanisms involved in tumorigenesis because autophagic cell death occurs in normal cells (e.g., fibroblasts or thymocytes) but not in most cancer cells. Furthermore, in some cancer cells, the magnitude of JNK activation, which is a crucial factor for autophagic cell death, is significantly lower compared with that in normal cells after exposure to apoptotic stimuli [21]. In these cancer cells, the JNK activity level may not reach the threshold level required to induce autophagic cell death. This conclusion is supported by evidence that the enforced expression of activated JNK in cancer cells induces autophagic cell death. Taken together, it is likely that insufficient activation of JNK followed by the failure of autophagic cell death may induce uncontrolled growth of cells, which ultimately acquire the malignant phenotype.
Autophagic Cell Death and Cancer Chemotherapeutics
Many molecularly targeted anticancer agents have been developed on the basis of apoptosis. However, autophagic cell death is also involved in the mechanisms of carcinogenesis. Therefore, we speculate that cancer may be cured by inducing autophagic death in cancer cells. This type of treatment would be effective for cancers that do not respond to existing anticancer agents (i.e., agents with apoptosis-based mechanisms of action). In addition, synergistic effects with existing anticancer agents are expected. Thus, we are now trying to develop molecularly targeted anticancer agents based on the induction of autophagic cell death. To meet this objective, we have established a high-throughput assay system that can monitor excessive autophagy and cell death–inducing activity. Using this assay, we screened a low molecular weight compound library to identify chemicals with autophagic cell death–inducing activities and successfully identified 24 candidate compounds. In particular, four of these compounds exhibited strong anticancer activities. At present, we are optimizing these compounds for development as drugs based on pharmacokinetic investigations and structure–activity relationships. In the near future, we aim to develop anticancer agents that will be effective against cancers that are resistant to the anticancer agents used in current clinical practice.
References
Tsujimoto Y (2003) Cell death regulation by the Bcl-2 protein family in the mitochondria. J Cell Physiol 195:158–167
Lindsten T, Ross AJ, King A et al (2000) The combined functions of proapoptotic Bcl-2 family members bak and bax are essential for normal development of multiple tissues. Mol Cell 6:1389–1399
Wei MC, Zong WX, Cheng EH et al (2001) Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730
Degterev A, Huang Z, Boyce M et al (2005) Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol 1:112–119
Shimizu S, Kanaseki T, Mizushima N et al (2004) A role of Bcl-2 family of proteins in nonapoptotic programmed cell death dependent on autophagy genes. Nat Cell Biol 6:1221–1228
Vandenabeele P, Galluzzi L, Vanden Berghe T et al (2010) Molecular mechanisms of necroptosis: an ordered cellular explosion. Nat Rev Mol Cell Biol 11:700–714
Mizushima N, Yoshimori T, Ohsumi Y (2011) The role of Atg proteins in autophagosome formation. Annu Rev Cell Dev Biol 27:107–132
Nakatogawa H, Suzuki K, Kamada Y et al (2009) Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nat Rev Mol Cell Biol 10:458–467
Kabeya Y, Kamada Y, Baba M et al (2005) Atg17 functions in cooperation with Atg1 and Atg13 in yeast autophagy. Mol Biol Cell 16:2544–2553
Axe EL, Walker SA, Manifava M et al (2008) Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182:685–701
Nishida Y, Arakawa S, Fujitani K et al (2009) Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461:654–658
Kundu M, Lindsten T, Yang CY et al (2008) Ulk1 plays a critical role in the autophagic clearance of mitochondria and ribosomes during reticulocyte maturation. Blood 112:1493–1502
Honda S, Arakawa S, Nishida Y et al (2014) Ulk1-mediated Atg5-independent macroautophagy mediates elimination of mitochondria from embryonic reticulocytes. Nat Commun 5. Article number:4004
Baehrecke EH (2003) Autophagic programmed cell death in Drosophila. Cell Death Differ 9:940–945
Qu X, Yu J, Bhagat G et al (2003) Promotion of tumorigenesis by heterozygous disruption of the beclin 1 autophagy gene. J Clin Invest 112:1809–1820
Yue Z, Jin S, Yang C et al (2003) Beclin 1, an autophagy gene essential for early embryonic development, is a haploinsufficient tumor suppressor. Proc Natl Acad Sci U S A 100:15077–15082
Iqbal J, Kucuk C, deLeeuw RJ et al (2009) Genomic analyses reveal global functional alterations that promote tumor growth and novel tumor suppressor genes in natural killer-cell malignancies. Leukemia 23:1139–1151
Huang X, Bai HM, Chen L et al (2010) Reduced expression of LC3B-II and Beclin 1 in glioblastoma multiforme indicates a down-regulated autophagic capacity that relates to the progression of astrocytic tumors. J Clin Neurosci 17:1515–1519
Mathew R, Karp CM, Beaudoin B et al (2009) Autophagy suppresses tumorigenesis through elimination of p62. Cell 137:1062–1075
Takamura A, Komatsu M, Hara T et al (2011) Autophagy-deficient mice develop multiple liver tumors. Genes Dev 25:795–800
Shimizu S, Konishi A, Nishida Y et al (2010) Involvement of JNK in the regulation of autophagic cell death. Oncogene 29:2070–2082
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Shimizu, S. (2015). Autophagic Cell Death and Cancer Chemotherapeutics. In: Nakao, K., Minato, N., Uemoto, S. (eds) Innovative Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55651-0_18
Download citation
DOI: https://doi.org/10.1007/978-4-431-55651-0_18
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55650-3
Online ISBN: 978-4-431-55651-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)