Abstract
Immune aging results in a decreased competence of adaptive immunity with an increased risk for autoimmunity. However, the mechanistic links between the immune aging and autoimmunity remain elusive. We reported that a PD-1+ memory phenotype (MP) CD4+ T cell population is increased as normal mice age, termed senescence-associated (SA-) T cells. The SA-T cells show characteristic signs and features of cellular senescence and emerge as follicular T cells in spontaneous germinal centers (GCs) that occur in aged mice. Spontaneous development of GCs is a hallmark of systemic autoimmune diseases, and we found that the development of SA-T cells is robustly and prematurely accelerated in bona fide lupus-prone mice in association with spontaneous, auto-reactive GCs. A fraction of the SA-T cells defined by CD153 expression is activated by autologous GC B cells to produce a plethora of inflammatory factors in a TCR-dependent manner and contributes to the expansion of the GC reactions, although the remaining part of them is rendered TCR anergic in situ. The results uncover that accelerated T cell senescence underlies the development of autoimmunity in systemic lupus erythematosus.
You have full access to this open access chapter, Download conference paper PDF
Similar content being viewed by others
Keywords
- Immune aging
- Systemic lupus erythematosus
- Cell senescence
- Senescence-associated T cells
- Follicular T cells
- Germinal centers
- Homeostatic proliferation
- Autoantibodies
Introduction: Immune Aging and Senescence-Associated T Cells
Immune aging (immunosenescence) is characterized by the reduced competence of acquired immunity, leading to increased susceptibility to infection as well as decreased vaccination efficiency. Recent accumulating evidence indicates that immunosenescence underlies an increased proinflammatory trait with age, including various chronic inflammatory and metabolic disorders, such as atherosclerosis and diabetes mellitus, as well as an increased risk for autoimmunity [1]. Cellular senescence is characterized by irreversible arrest of proliferation, grossly altered gene expression, and relative resistance to apoptosis [2]. Notably, senescent cells are often metabolically active and may become foci of host reactions in tissues by secreting various inflammatory factors [3]. The features and consequences of cellular senescence in T cells in the immune system, however, remain elusive.
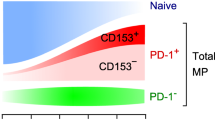
One of the most prominent changes occurring in the immune organs with age is an early involution of the thymus. The thymus is a central immune organ to support T cell development and establish T cell self-tolerance. The T cell generation in the thymus is most active at the late embryonic through neonatal stages, but the activity sharply declines after the juvenile stage, eventually replaced almost entirely by fat tissues at later stages of life (Fig. 1a). Although the mechanism of early thymic involution remains elusive, it is suggested that the rapid decrease of thymic epithelial stem cell (TECSC) activity after birth nay play a crucial role in it [4]. In concordance with the decrease of neo-T cell genesis, the peripheral naïve T cells are gradually reduced with age. Although the peripheral T cell pool is well maintained in aged individuals, the population shows a steady increase in the proportions of memory phenotype (MP) T cells [5]. It is suggested that these MP T cells include those that have homeostatically expanded in response to the decreasing drift of the T cell numbers, in addition to the authentic memory T cells for specific environmental antigens [5]. We reported that a unique PD-1+ MP CD4+ T cell population is increased with age [6], now termed senescence-associated (SA-) T cells (Fig. 1b). The SA-T cells showed compromised capacity of clonal proliferation and production of typical T cell–specific cytokines via TCR-stimulation; however, the SA-T cells secrete abundant proinflammatory cytokines such as osteopontin (OPN) [6].
SA-T Cells Represent an Endogenously Arising Follicular T Cell Population with Age
We found that normal aged mice often developed GCs spontaneously in the secondary lymphoid tissues, and the SA-T cells were detected mostly in the GCs, representing follicular T cells (Fig. 2). In agreement with the follicular localization, the majority of SA-T cells expressed ICOS and CD200, similarly to the typical follicular helper T cells induced by immunization with exogenous antigens such as sheep red blood cells (SRBC-TFH). However, a significant portion of the SA-T cells additionally expressed CD153 (CD30L), which was rarely expressed in SRBC-TFH cells. Functionally, CD153+ SA-T cells showed compromised proliferation but produced large amounts of OPN via TCR-stimulation with much less IL-4 and IFN-γ, whereas the rest of the CD153− population did not proliferate or produce any of these cytokines at all, suggesting that these cells were TCR unresponsive. The SA-T cells showed a unique transcriptome (Fig. 3) different from those of PD-1 MP CD4+ T cells and SRBC-TFH cells, but the CD153+ and CD153− fractions of the SA-T cells had very similar transcriptome profiles to each other, despite the distinct TCR responsiveness. The results suggest that SA-T cells represent a unique type of follicular CD4+ T cell population, a fraction of which is rendered TCR anergic in situ. Although CD153+ and CD153− SA-T cells became detectable only in normal aged mice, the development was remarkably accelerated in the adult-thymectomized mice or γ-ray irradiated with transplanted naïve T cells. The results strongly suggest that the SA-T cells are generated in the process of homeostatic T cell proliferation, either acutely or chronically depending on the extent and tempo of the decrease in a peripheral T cell pool.
SA-T Cells Increase Robustly in Bone Fide Lupus-Prone Mice
Spontaneous GC reactions are a hallmark of systemic autoimmune diseases, including lupus [7, 8], and the findings above prompted us to examine them in female BWF1 mice, a bona fide mouse model of human SLE. Female BWF1 mice robustly developed GCs with a remarkable increase of follicular PD-1+ MP CD4+ T cells beginning from as early as 6 months of age (Fig. 4a); this was not the case in male BWF1 mice or parental NZW mice. A significant portion of them expressed CD153 in addition to other follicular T cell markers such as CXCR5, ICOS, and Bcl-6 (Fig. 4b). Transcriptome analysis confirmed that both CD153+ and CD153− cell fractions showed highly coincidental profiles with the corresponding SA-T cells in normal aged mice, rather than SRBC-TFH cells. Moreover, the TCR-stimulated CD153+ cell fraction produced abundant OPN in addition to other inflammatory chemokines (Ccl1, Ccl3, Ccl4) with minimal production of T cell–specific cytokines, whereas the CD153− fraction produced no cytokines at all, including OPN. Overall, the follicular T cells in female BWF1 mice showed features essentially identical to those of the SA-T cells in normal aged mice, except for more robust and earlier development.
SA-T Cells Show Typical Signs and Features of Cellular Senescence
Transcriptome profiles of the SA-T cells of both aged B6 and female BWF1 mice revealed the up-regulation of a numbers of genes coding for potentially inflammatory factors including Spp1 (Fig. 5a). The feature was reminiscent of a so-called senescence-associated secretory phenotype (SASP) [3], and indeed the expression of Cebpb, shown to be involved in the regulation of SASP, was also increased. In addition, the SA-T cells revealed increased expression of Cdkn1a (Cip1) and Cdkn2b (Ink4b) (Fig. 5b), which are representative senescence-associated biomarkers [9, 10], remarkably increased nuclear heterochromatin foci, and higher SA-β-Gal activity than PD-1− CD4+ T cells (Fig. 5c). Also, the SA-T cells were highly stable in culture and survived for long terms without proliferation. All of these results are consistent with the notion that the SA-T cells represent the T cells in cellular senescence.
SA-T Cells Are Activated in Response to Autologous GC B Cells to Produce OPN that Protects Against B Cell Apoptosis in GCs
The Spp1 transcripts were sharply increased in the spleens of female BWF1 mice from around 5 months of age, in concordance with the overt GC reactions. Analysis with laser-capture microdissection (LMD) revealed that the Spp1 was confined to the PNA+ GC regions, whereas the GCs in SRBC-immunized B6 mice hardly expressed Spp1. When SA-T cells from female BWF1 mice were co-cultured with γ-ray-irradiated autologous B cells, the CD153+, but hardly TCR-anergic CD153−, fraction produced significant amounts of OPN. The response was induced by CD95+ GC B cells more efficiently than CD95− B cells and was inhibited significantly in the presence of anti-MHC II or soluble anti-CD3 antibody, suggesting that the SA-T cells recognized self-antigens presented by GC B cells via TCR. Curiously, however, the response was unaffected in the presence of anti-PD-L2 antibody, albeit that the GC-B cells expressed PD-L2, suggesting that the OPN production by CD153+ SA-T cells is mediated by a PD-1-resistant, alternative TCR-signaling pathway (Fig. 6). On the other hand, the OPN production in response to GC-B cells was significantly enhanced by CD30-Ig agonistic to CD153 (Fig. 6). The primary B cells from female BWF1 mice showed significant cell death from stimulation with anti-μ antibody. However, OPN prevented B cell death and accordingly enhanced the secretion of anti-dsDNA antibody in vitro. Upon analysis using a WEHI231 B1 cell line, it was indicated that OPN inhibited the BCR-induced apoptosis by inducing the expression of Bcl2a1a, which is known to be crucial for B cell survival (Fig. 6). The results suggest that the SA-T cells specifically recognize GC-B cells and locally produce OPN, which in turn protects the GC B cells from antigen-induced apoptosis.
CD153+ SA-T cells specifically respond to own GC-B cells and produce abundant OPN, which prevent the B cells from BCR-induced apoptosis. The TCR-signaling pathway is enhanced via CD153 co-stimulatory activity but is unaffected by negative PD-1 signaling. The CD153+ SA-T cells may eventually be rendered TCR anergic via a tolerance checkpoint and become CD153− cells
Involvement of SA-T Cells in the Expansion of GCs and Autoimmunity in Lupus
To directly investigate the role of SA-T cells in GC reaction, we isolated PD-1− MP CD4+ and SA-T cells from female BWF1 mice with overt lupus disease and transferred them into pre-disease young BWF1 mice. We found that the CD153+ SA-T cells caused a significant increase in GC sizes in the recipients, whereas the PD-1− MP CD4+ cells barely affected the GC reactions. Moreover, a partial depletion of CD153+ SA-T cells in female BWF1 mice at 4 months of age by treatment with a rat anti-CD153 antibody (500 μg/head) twice a week for 4 weeks resulted in significant reduction of GC sizes as well as BC-B cells; none of these mice developed significant anti-dsDNA antibody, whereas 20 % (3 out of 15) of control mice showed a burst of anti-dsDNA antibody. These results suggest that CD153+ SA-T cells are directly involved in the growth of spontaneous GCs and autoantibody production in female BWF1 mice.
Conclusion and Perspectives
Among a number of changes in immune function with age is an increasing risk for autoimmunity [1]. We previously proposed that the increase of SA-T cells chronically with age and acutely with a leukemic condition might be related to the decrease of T cell genesis due to physiological thymic involution and leukemia-associated T-lymphocytopenia, respectively [6]. In a lymphopenic condition, the peripheral CD4+ T cells undergo homeostatic proliferation, which depends on homeostatic cytokines and tonic TCR-signal via MHC II–bearing self-peptides [5]. As such, the sustained CD4+ T cell homeostatic proliferation may lead to an increased risk for autoimmunity [11]. It was reported that CD4+ T cell homeostatic proliferation underlies the development of autoimmune diabetes in female NOD mice [12]. Lymphopenia is one of the characteristic features in lupus disease, and therefore we propose that CD4+ T cell homeostatic proliferation underlies the robust and premature development of SA-T cells. A recent report suggests that homeostatically proliferating PD-1+ CD4+ T cells may show different energy metabolism from CD4+ T cells driven by antigens in immune response [13]. Homeostatic proliferation is not associated with the transition to the effector phase as in immune response, and the possibility that the persistence of such a proliferative state with particular energy metabolism leads to a higher propensity for the progression of cellular senescence would be intriguing (Fig. 7). In conclusion, our current results indicate that SA-T cells play an important part in lupus pathogenesis and may provide a novel therapeutic clue for controlling human SLE.
Normal naïve CD4+ T cells are activated in response to the specific foreign antigens presented by professional antigen-presenting cells (APCs), such as DCs, and show a robust clonal expansion, followed by maturation to the effector cells or quiescent memory cells. In lymphopenic conditions, however, naïve CD4+ T cells proliferate polyclonally, called homeostatic proliferation, which depends on the APCs such as B cells presenting self-peptides and homeostatic cytokines, to maintain the peripheral T cell pool. The sustained homeostatic proliferation may result in an increased risk for autoimmunity as well as T cell senescence to become SA-T cells. The (CD153+) SA-T cells are quite stable and metabolically active, producing abundant inflammatory factors such as OPN and chemokines, and become inflammatory foci in tissues. In the lupus-prone genetic background such as BWF1 with a defective B cell self-tolerance checkpoint, the SA-T cells are involved in auto-reactive GC reactions and promote overt autoantibody production
References
Goronzy JJ, Weyand CM (2012) Immune aging and autoimmunity. Cell Mol Life Sci 69(10):1615–1623
Campisi J, d’Adda d, Fagagna F (2007) Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8(9):729–740
Freund A, Orjalo AV, Desprez PY, Campisi J (2010) Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med 16(5):238–246
Chinn IK et al (2012) Changes in primary lymphoid organs with aging. Semin Immunol 24:309–320
Sprent J, Surh CD (2011) Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol 12(6):478–484
Shimatani K, Nakashima Y, Hattori M, Hamazaki Y, Minato N (2009) PD-1+ memory phenotype CD4+ T cells expressing C/EBPalpha underlie T cell immunodepression in senescence and leukemia. Proc Natl Acad Sci U S A 106(37):15807–15812
Vinuesa CG, Sanz I, Cook MC (2009) Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol 9(12):845–857
Grammer AC et al (2003) Abnormal germinal center reactions in systemic lupus erythematosus demonstrated by blockade of CD154-CD40 interactions. J Clin Invest 112(10):1506–1520
Kim WY, Sharpless NE (2006) The regulation of INK4/ARF in cancer and aging. Cell 127(2):265–275
Krishnamurthy J et al (2004) Ink4a/Arf expression is a biomarker of aging. J Clin Invest 114(9):1299–1307
Baccala R, Theofilopoulos AN (2005) The new paradigm of T-cell homeostatic proliferation-induced autoimmunity. Trends Immunol 26(1):5–8
King C, Ilic A, Koelsch K, Sarvetnick N (2004) Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell 117(2):265–277
Chang CH et al (2013) Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 153(6):1239–1251
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is distributed under the terms of the Creative Commons Attribution Noncommercial License, which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Copyright information
© 2015 The Author(s)
About this paper
Cite this paper
Minato, N. (2015). T Cell Senescence and Autoimmunity. In: Nakao, K., Minato, N., Uemoto, S. (eds) Innovative Medicine. Springer, Tokyo. https://doi.org/10.1007/978-4-431-55651-0_10
Download citation
DOI: https://doi.org/10.1007/978-4-431-55651-0_10
Publisher Name: Springer, Tokyo
Print ISBN: 978-4-431-55650-3
Online ISBN: 978-4-431-55651-0
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)