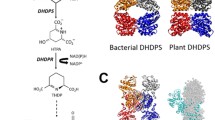
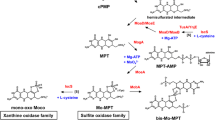
Summary
Methylamine oxidase (MAOX) from Gram-positive soil bacterium Arthrobacter PI catalyzes the oxidation of CH3NH2 to H2C=O and NH4 + via reduction of O2 to H2O2. Past work indicates that MAOx is similar to mammalian plasma amine oxidase (PAO) and diamine oxidase (DAO), plant DAO, and yeast peroxisomal amine oxidase (YAO). All have Mr ≃ 170,000 and are composed of 2 identical subunits, each of which contains 1 atom of Cu(II) and one molecule of quinonoid cofactor. Herein, we report further evidence as to the striking similarity of these enzymes, and describe properties of MAOX which offer insights into understanding the eukaryotic oxidases. It is our belief that the structure of the quinone cofactor, and the Cu(II) site in MAOX are identical to these sites in PAO and DAO.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Preview
Unable to display preview. Download preview PDF.
Similar content being viewed by others
References
Bruinenberg PG, Evers M, Waterham HR, Kuipers J, Arnberg AC, Greet AB (1989) Cloning and sequencing of the peroxisomal amine oxidase from Hansenula polymorpha. Biochim Biophys Acta 1008:157–167.
Dooley DM, McIntire WS, McGuirl MA, Cote CE, Bates JL (1990) Characterization of the active site of Arthrobacter P1 methylamine oxidase: evidence for cooper-quinone interactions. J Am Chem Soc 112:2782–2789.
Finazzi-Argo A, Rinaldi A, Floris G, Rotilio G (1984) A free-radical intermediate in the reduction of plant Cu-amine oxidases. FEBS Lett 176:378–380.
Hartmann C, Klinman JP (1988) Pyrroloquinoline quinone: a new redox cofactor in eukaryotic enzymes. BioFactors 1:41–49.
Hartmann C, Klinman JP (1987) Reductive trapping of substrate to bovine plasma amine oxidase. J Biol Chem 262:962–965.
Hartmann C, Klinman P (1990) Reductive trapping of substrate to methylamine oxidase from Arthrobacter P1. FEBS Lett 261:441–444.
Knowles PF, Pandeya KB, Rius FX, Spencer CM, Moog RS, McGuirl MA, Dooley DM (1987) The organic cofactor in plasma amine oxidase: evidence for pyrroloquinoline quinone and against pyridoxal phosphate. Biochem J 241:603–608.
McCracken J, Peisach J, Dooley DM (1987) Cu(II) coordination chemistry of amine oxidases: pulsed EPR studies of histidine imidazole, water, and exogenous ligand coordination. J Am Chem Soc 109:4064–4072.
Morpurgo L, Befani O, Sabatini S, Mondavi B, Artioc M, Corello F, Massa S, Stefancich G, Avigliano L (1988) Spectroscopic studies of the reaction between bovine serum amine oxidase (copper-containing) and some hydrazides and hydrazines. Biochem J 256:565–570.
Rinaldi A, Giartosio A, Floris G, Medda R, Finazzi-Argo A (1984) Lentil seedling amine oxidase: preparation and properties of the copper-free enzyme. Biochem Biophys Res Commun 120:245–294.
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 1990 Springer-Verlag
About this paper
Cite this paper
McIntire, W.S., Dooley, D.M., McGuirl, M.A., Cote, C.E., Bates, J.L. (1990). Methylamine oxidase from Arthrobacter P1 as a prototype of eukaryotic plasma amine oxidase and diamine oxidase. In: Riederer, P., Youdim, M.B.H. (eds) Amine Oxidases and Their Impact on Neurobiology. Journal of Neural Transmission, vol 32. Springer, Vienna. https://doi.org/10.1007/978-3-7091-9113-2_40
Download citation
DOI: https://doi.org/10.1007/978-3-7091-9113-2_40
Publisher Name: Springer, Vienna
Print ISBN: 978-3-211-82239-5
Online ISBN: 978-3-7091-9113-2
eBook Packages: Springer Book Archive